394-47-8
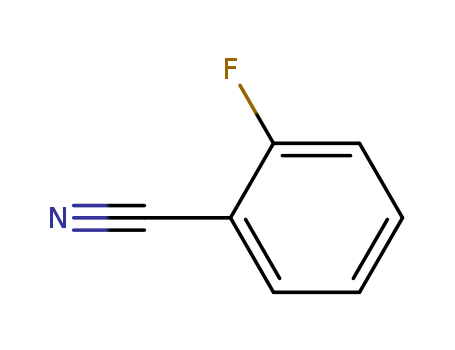
- Product Name:2-fluorobenzonitrile
- Molecular Formula:C7H4FN
- Purity:99%
- Molecular Weight:121.114
Product Details
Bulk supply high purity 2-fluorobenzonitrile 394-47-8, Paid sample available
- Molecular Formula:C7H4FN
- Molecular Weight:121.114
- Appearance/Colour:colorless to light yellow liquid
- Vapor Pressure:0.46mmHg at 25°C
- Melting Point:35 - 37oC
- Refractive Index:1.5089
- Boiling Point:193.6 °C at 760 mmHg
- Flash Point:73.9 °C
- PSA:23.79000
- Density:1.15 g/cm3
- LogP:1.69738
2-Fluorobenzonitrile(Cas 394-47-8) Usage
|
Synthesis |
Tetramethylammonium fluoride (TMAF) has been found to be an effective fluorodenitration reagent. The good selectivity for the fluoroaromatic and not the phenol has been attributed to the stability of ion pairing between nitrite and tetramethylammonium ions. TMAF reacts with 2-nitrobenzonitrile to give a quantitative conversion to 2-fluorobenzonitrile. |
|
General Description |
2-Fluorobenzonitrile reacts with lithium N,N-dialkylaminoborohydride reagent to yield 2-(N,N-dialkylamino)benzylamines. |
InChI:InChI=1/C7H4FN/c8-7-4-2-1-3-6(7)5-9/h1-4H
394-47-8 Relevant articles
Visible Light Generation of a Microsecond Long-Lived Potent Reducing Agent
Hu, Ke,Li, Pengju,Meyer, Gerald J.,Niu, Fushuang,Wang, Hanqi,Zhang, Zhenghao,Zhao, Zijian
supporting information, (2022/03/27)
Photoexcitation of molecular radicals ca...
Method for catalyzing oxidation of amines to generate nitrile by using nonmetal mesoporous nitrogen-doped carbon material
-
Paragraph 0019; 0037, (2021/05/08)
The invention discloses a method for pre...
Tetramethylammonium Fluoride Alcohol Adducts for SNAr Fluorination
Bland, Douglas C.,Lee, So Jeong,Morales-Colón, Mariá T.,Sanford, Melanie S.,Scott, Peter J. H.,See, Yi Yang
supporting information, p. 4493 - 4498 (2021/06/28)
Nucleophilic aromatic fluorination (SNAr...
Method for efficiently synthesizing fluorine-containing compound
-
Paragraph 0036-0054, (2021/06/26)
The invention discloses a method for eff...
394-47-8 Process route
-
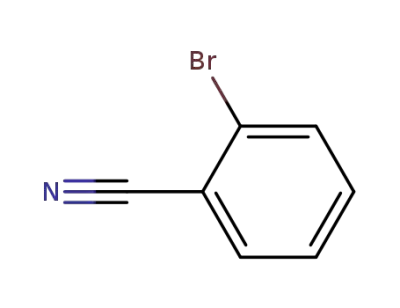
-
2042-37-7
o-cyanobromobenzene

-
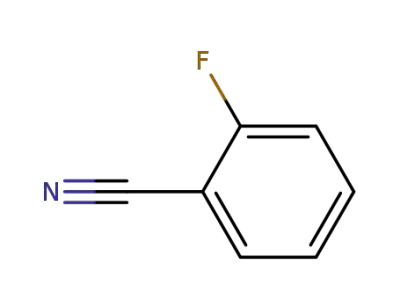
-
394-47-8
2-fluorobenzonitrile

-

-
100-47-0
benzonitrile
| Conditions | Yield |
|---|---|
|
o-cyanobromobenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -28 ℃;
Flow reactor;
With
N-fluorobis(benzenesulfon)imide;
In
tetrahydrofuran; hexane;
at -28 ℃;
Reagent/catalyst;
Flow reactor;
|
79% 6% |
-
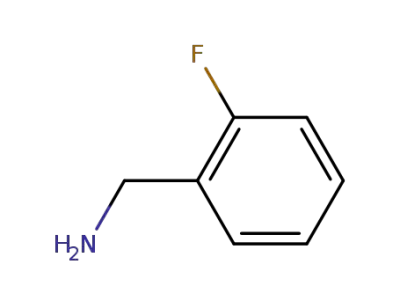
-
89-99-6
2-fluorobenzylamine

-

-
394-47-8
2-fluorobenzonitrile

-
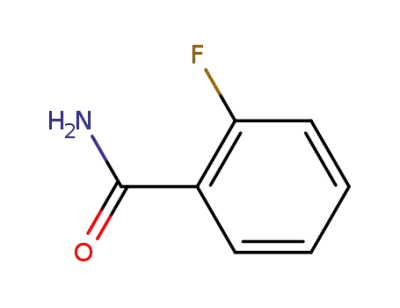
-
445-28-3
2-fluorobenzamide
| Conditions | Yield |
|---|---|
|
With
ammonium hydroxide; oxygen;
In
tert-Amyl alcohol;
at 110 ℃;
for 16h;
under 1500.15 Torr;
|
60.8% 23% |
394-47-8 Upstream products
-
445-28-3

2-fluorobenzamide
-
462-06-6
fluorobenzene
-
460-19-5
ethanedinitrile
-
873-32-5
2-Chlorobenzonitrile
394-47-8 Downstream products
-
342-22-3
(2-fluoroxyphenyl)(o-tolyl)methanone
-
446-22-0
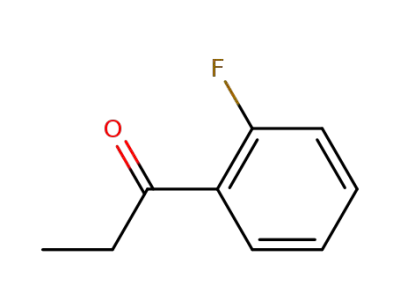
1-(2-fluorophenyl)propan-1-one
-
131269-82-4
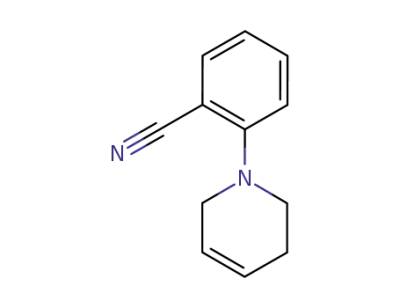
2-(1,2,3,6-Tetrahydropyridin-1-yl)-benzonitril
-
25699-87-0
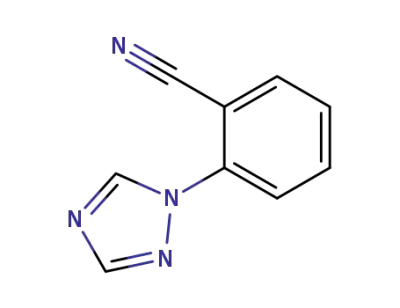
2-(1',2',4'-triazol-1'-yl)benzonitrile
Relevant Products
-
5-Chloro-2-fluoropyridine
CAS:1480-65-5
-
Ethyl acetate
CAS:141-78-6
-
Isopropanol
CAS:67-63-0