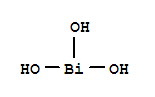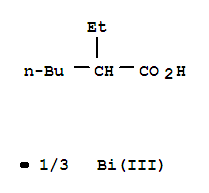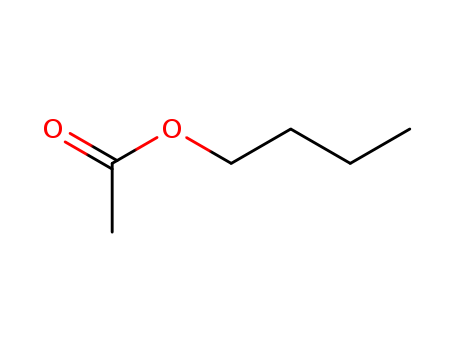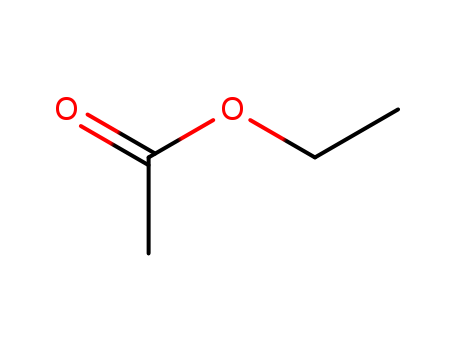
141-78-6
- Product Name:Ethyl acetate
- Molecular Formula:C4H8O2
- Purity:99%
- Molecular Weight:88.1063
Product Details
Bulk supply high purity Ethyl acetate 141-78-6, Paid sample available
- Molecular Formula:C4H8O2
- Molecular Weight:88.1063
- Appearance/Colour:colorless liquid
- Vapor Pressure:73 mm Hg ( 20 °C)
- Melting Point:-83.6 °C, 190 K, -118 °F
- Refractive Index:n20/D 1.3720(lit.)
- Boiling Point:77.1 °C, 350 K, 171 °F
- PKA:16-18(at 25℃)
- Flash Point:-4 °C
- PSA:26.30000
- Density:0.898 g/cm3
- LogP:0.56940
Ethyl acetate(Cas 141-78-6) Usage
|
Chemical Description |
Ethyl acetate is a colorless liquid used as a solvent. |
|
organic ester compound |
Ethyl Acetate is an organic ester compound with a molecular formula of C4H8O2 (commonly abbreviated as EtOAc or EA), appears as a colorless liquid. It is highly miscible with all common organic solvents (alcohols, ketones, glycols, esters), which make it a common solvent for cleaning, paint removal and coatings. Ethyl acetate is found in alcoholic beverages, cereal crops, radishes, fruit juices, beer, wine, spirits etc. It has a fruity characteristic odor that is commonly recognized in glues, nail polish remover, decaffeinating tea and coffee, and cigarettes. Due to its agreeable aroma and low cost, this chemical is commonly used and manufactured in large scale in the world, as over 1 million tons annually. ethyl acetate structure |
|
Purification and water removal methods |
Ethyl acetate generally has a content of 95% to 98% containing a small amount of water, ethanol and acetic acid. It can be further purified as following: add 100mL of acetic anhydride into 1000mL of ethyl acetate; add 10 drops of concentrated sulfuric acid, heat and reflux for 4h to remove impurities such as ethanol and water, and then further subject to distillation. Distillate is oscillated by 20~30g of anhydrous potassium carbonate and further subject to re-distillation. The product has a boiling point of 77 °C and purity being over 99%. |
|
Production |
Industrial production of ethyl acetate is mainly classified into three processes. The first one is a classical Fischer esterification process of ethanol with acetic acid in presence of acid catalyst. This process needs acid catalyst2 such as sulphuric acid, hydrochloride acid, ptoluene sulfonic acid etc. This mixture converts to the ester in about 65% yield at room temperature.? CH3CH2OH + CH3COOH ? CH3COOC2H5 + H2O The reaction can be accelerated by acid catalysis and the equilibrium can be shifted to the right by removal of water. The second one is Tishchenko Reaction of acetaldehyde using aluminium triethoxide as a catalyst. In Germany and Japan, most ethyl acetate is produced via the Tishchenko process.? 2 CH3CHO → CH3COOC2H5 This method has been proposed by two different routes; (i) dehydrogenative process, which uses copper or palladium based catalyst and (ii) the oxidative one, which employs, PdO supported catalysts. The third one, which has been recently commercialized, is addition of acetic acid to ethylene using clay and heteroploy acid7 as a catalyst.? CH2= CH2 + CH3COOH → CH3COOC2H5? The processes, however, have some disadvantages; both the conventional esterification and addition of acetic acid to ethylene need stock tanks and apparatus for several feed stocks. Moreover, they use acetic acid that causes apparatus corrosion. Although Teshchenko Reaction uses only one feed and it is a non-corrosive material, it is difficult to handle acetaldehyde because is not available outside of petrochemical industrial area. In such circumstances, an improved process of ethyl acetate production is strongly desired. |
|
Extinguishing agent |
dry powder, dry sand, carbon dioxide, foam, and 1211 fire extinguishing agent |
|
Professional standards |
TWA 1400 mg/m3; STEL 2000 mg/m3 |
|
Physical properties |
Clear, colorless, mobile liquid with a pleasant, sweet fruity odor. Experimentally determined detection and recognition odor threshold concentrations were 23 mg/m3 (6.4 ppmv) and 48 mg/m3 (13.3 ppmv), respectively (Hellman and Small, 1974). Cometto-Mu?iz and Cain (1991) reported an average nasal pungency threshold concentration of 67,300 ppmv. |
|
Production Methods |
Ethyl acetate can be manufactured by the slow distillation of a mixture of ethanol and acetic acid in the presence of concentrated sulfuric acid. It has also been prepared from ethylene using an aluminum alkoxide catalyst. |
|
Preparation |
Ethyl acetate is made by esterification of acetic acid with ethanol, from acetaldehyde, or by the direct addition of ethylene to acetic acid. BP started a 220,000 tonne/year plant in 2001 to operate the last of these processes, known as AVADA. Ethylene and acetic acid react in the presence of a heteropolyacid catalyst to give ethyl acetate at a claimed high selectivity and 99.97% purity. This is the world’s largest ethyl acetate plant and is motivated by its increasing use as a more “acceptable” solvent than hydrocarbons. In some countries, where ethanol is expensive or there is surplus acetaldehyde capacity, ethyl acetate is made by a Tishchenko reaction. Sasol in South Africa was said to be investigating such a process in the early 2000s. Ethanol is a solvent for surface coatings, cleaning preparations, and cosmetics. Industrial ethanol is aerobically fermented to white vinegar (dilute acetic acid) of the type used for pickling. Gourmet vinegars—wine vinegar, cider vinegar, and so on, made by fermentation of alcoholic beverages—are also available. Ten percent of industrial ethanol production was used for vinegar in the United States in 2001. |
|
Reactions |
Ethyl acetate can be hydrolyzed in acidic or basic conditions to regain acetic acid and ethanol. The use of an acid catalyst accelerates the hydrolysis, which is subject to the Fischer equilibrium mentioned above. In the laboratory, and usually for illustrative purposes only, ethyl esters are typically hydrolyzed in a two step process starting with a stoichiometric amount of strong base, such as sodium hydroxide. This reaction gives ethanol and sodium acetate, which is unreactive toward ethanol: CH3CO2C2H5 + Na OH → C2H5OH + CH3CO2Na The rate constant is 0.111 dm3 / mol.sec at 25 °C. |
|
Aroma threshold values |
Detection: 5 ppb to 5 ppm |
|
General Description |
Ethyl acetate, a carboxylate ester, is bio-friendly organic solvent with wide range of industrial applications. Its synthesis by reactive distillation and by acceptorless dehydrogenative dimerization of ethanol has been explored. Its utility as a less toxic alternative to diethyl ether in the formalin-ether (F-E) sedimentation procedure for intestinal parasites has been investigated. Its ability as an acyl acceptor in the immobilized lipase-mediated preparation of biodiesel from crude vegetable oils has been examined. The complete degradation of ethyl acetate to CO2 using manganese octahedral molecular sieve (OMS-2) has been investigated. |
|
Air & Water Reactions |
Highly flammable. Slightly soluble in water. Ethyl acetate is slowly hydrolyzed by moisture. |
|
Reactivity Profile |
Ethyl acetate is also sensitive to heat. On prolonged storage, materials containing similar functional groups have formed explosive peroxides. Ethyl acetate may ignite or explode with lithium aluminum hydride. Ethyl acetate may also ignite with potassium tert-butoxide. Ethyl acetate is incompatible with nitrates, strong alkalis and strong acids. Ethyl acetate will attack some forms of plastics, rubber and coatings. Ethyl acetate is incompatible with oxidizers such as hydrogen peroxide, nitric acid, perchloric acid and chromium trioxide. Violent reactions occur with chlorosulfonic acid. . SOCl2 reacts with esters, such as Ethyl acetate, forming toxic SO2 gas and water soluble/toxic acyl chlorides, catalyzed by Fe or Zn (Spagnuolo, C.J. et al. 1992. Chemical and Engineering News 70(22):2.). |
|
Health Hazard |
The acute toxicity of ethyl acetate is low. Ethyl acetate vapor causes eye, skin, and respiratory tract irritation at concentrations above 400 ppm. Exposure to high concentrations may lead to headache, nausea, blurred vision, central nervous system depression, dizziness, drowsiness, and fatigue. Ingestion of ethyl acetate may cause gastrointestinal irritation and, with larger amounts, central nervous system depression. Eye contact with the liquid can produce temporary irritation and lacrimation. Skin contact produces irritation. Ethyl acetate is regarded as a substance with good warning properties. No chronic systemic effects have been reported in humans, and ethyl acetate has not been shown to be a human carcinogen, reproductive, or developmental toxin |
|
Flammability and Explosibility |
Ethyl acetate is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Ethyl acetate vapor forms explosive mixtures with air at concentrations of 2 to 11.5% (by volume). Hazardous gases produced in ethyl acetate fires include carbon monoxide and carbon dioxide. Carbon dioxide or dry chemical extinguishers should be used for ethyl acetate fires. |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
Pharmaceutical Applications |
In pharmaceutical preparations, ethyl acetate is primarily used as a solvent, although it has also been used as a flavoring agent. As a solvent, it is included in topical solutions and gels, and in edible printing inks used for tablets.Ethyl acetate has also been shown to increase the solubility of chlortalidone and to modify the polymorphic crystal forms obtained for piroxicam pivalate, mefenamic acid, and fluconazole,and has been used in the formulation of microspheres. Ethyl acetate has been used as a solvent in the preparation of a liposomal amphotericin B dry powder inhaler formulation.(9) Its use as a chemical enhancer for the transdermal iontophoresis of insulin has been investigated. In food applications, ethyl acetate is mainly used as a flavoring agent. It is also used in artificial fruit essence and as an extraction solvent in food processing. |
|
Safety Profile |
Potentially poisonous by ingestion. Toxicity depends upon alcohols in question, generally ethanol with methanol as a denaturant. A flammable liquid and dangerous fire hazard; can react vigorously with oxidzing materials. Moderate explosion hazard. See ETHANOL, METHYL ALCOHOL, and n-PROPYL ALCOHOL. |
|
Safety |
Ethyl acetate is used in foods, and oral and topical pharmaceutical formulations. It is generally regarded as a relatively nontoxic and nonirritant material when used as an excipient. However, ethyl acetate may be irritant to mucous membranes, and high concentrations may cause central nervous system depression. Potential symptoms of overexposure include irritation of the eyes, nose, and throat, narcosis, and dermatitis. Ethyl acetate has not been shown to be a human carcinogen or a reproductive or developmental toxin. The WHO has set an estimated acceptable daily intake of ethyl acetate at up to 25 mg/kg body-weight. In the UK, it has been recommended that ethyl acetate be temporarily permitted for use as a solvent in food and that the maximum concentration consumed in food should be set at 1000 ppm. LD50 (cat, SC): 3.00 g/kg LD50 (guinea-pig, oral): 5.50 g/kg LD50 (guinea-pig, SC): 3.00 g/kg LD50 (mouse, IP): 0.709 g/kg LD50 (mouse, oral): 4.10 g/kg LD50 (rabbit, oral): 4.935 g/kg LD50 (rat, oral): 5.62 g/kg |
|
Synthesis |
By reacting acetic acid and ethanol in the presence of sulfuric acid; by distillation of sodium potassium, or lead acetate with ethanol in the presence of sulfuric acid; by polymerizatin of acetaldehyde in the presence of aluminum ethylate or aluminum acetate as catalysts. |
|
Potential Exposure |
This material is used as a solvent for nitrocellulose and lacquer. It is also used in making dyes,flavoring and perfumery, and in smokeless powder manufacture |
|
Carcinogenicity |
Ethyl acetate was not mutagenic in bacterial assays; it was not genotoxic in a number of in vivo assays but did cause chromosomal damage in hamster cells in vitro. Ethyl acetate has a fruity odor detectable at 10ppm. The 2003 ACGIH threshold limit valuetime- weighted average (TLV-TWA) for ethyl acetate is 400pm (1440mg/m3). |
|
Environmental fate |
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.00 g/g which is 54.9% of the ThOD value of 1.82 g/g. Photolytic. Reported rate constants for the reaction of ethyl acetate and OH radicals in the atmosphere (296 K) and aqueous solution are 1.51 x 10-12 and 6.60 x 10-13 cm3/molecule?sec, respectively (Wallington et al., 1988b). Chemical/Physical. Hydrolyzes in water forming ethanol and acetic acid (Kollig, 1993). The estimated hydrolysis half-life at 25 °C and pH 7 is 2.0 yr (Mabey and Mill, 1978). |
|
Metabolism |
Ethyl acetate is hydrolysed to ethyl alcohol, which is then partly excreted in the expired air and urine. The rest is metabolized, the acetate fraction becoming incor porated in the body pool (Fassett, 1963). |
|
storage |
Ethyl acetate should be stored in an airtight container, protected from light and at a temperature not exceeding 30°C. Ethyl acetate is slowly decomposed by moisture and becomes acidic; the material can absorb up to 3.3% w/w water.Ethyl acetate decomposes on heating to produce ethanol and acetic acid, and will emit acrid smoke and irritating fumes. It is flammable and its vapor may travel a considerable distance to an ignition source and cause a ‘flashback’. The alkaline hydrolysis of ethyl acetate has been shown to be inhibited by polyethylene glycol and by mixed micelle systems. |
|
Shipping |
UN1173 Ethyl acetate, Hazard Class: 3; Labels: 3-Flammable liquid. |
|
Purification Methods |
The most common impurities in EtOAc are water, EtOH and acetic acid. These can be removed by washing with aqueous 5% Na2CO3, then with saturated aqueous CaCl2 or NaCl, and drying with K2CO3, CaSO4 or MgSO4. More efficient drying is achieved if the solvent is further dried with P2O5, CaH2 or molecular sieves before distillation. CaO has also been used. Alternatively, ethanol can be converted to ethyl acetate by refluxing with acetic anhydride (ca 1mL per 10mL of ester), the liquid is then fractionally distilled, dried with K2CO3 and redistilled. [Beilstein 2 III 127.] |
|
Toxicity evaluation |
Ethyl acetate is rapidly hydrolyzed to ethanol and acetic acid. When ethyl acetate was injected intraperitoneal at 1.6 g kg-1, hydrolysis to acetic acid and ethanol occurred rapidly. The biological half-life value of the conversion of ethyl acetate to ethanol was found to be between 5 and 10 min. At doses higher than 1.6 g kg-1 in rats the rate of hydrolysis exceeded the ethanol oxidation leading to the ethanol accumulation in the vascular system. |
|
Incompatibilities |
Ethyl acetate can react vigorously with strong oxidizers, strong alkalis, strong acids, and nitrates to cause fires or explosions. It also reacts vigorously with chlorosulfonic acid, lithium aluminum hydride, 2-chloromethylfuran, and potassium tert-butoxide. |
|
Waste Disposal |
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (oral tablets and sustained-action tablets; topical and transdermal preparations). Included in nonparenteral medicines licensed in the UK (tablets, topical solutions, and gels). Ethyl acetate is also accepted for use in food applications in a number of countries including the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
InChI:InChI=1/C4H8O2/c1-3-6-4(2)5/h3H2,1-2H3
141-78-6 Relevant articles
-
Lane et al.
, p. 6492 (1968)
-
Activated carbon aerogel supported copper catalysts for the hydrogenation of methyl acetate to ethanol: Effect of KOH activation
Hou, Xiaoxiong,Zhao, Jinxian,Liu, Junjie,Han, Yahong,Pei, Yongli,Ren, Jun
, p. 9430 - 9438 (2019)
Methyl acetate (MA) hydrogenation is cru...
ESI-MS Insights into Acceptorless Dehydrogenative Coupling of Alcohols
Vicent, Cristian,Gusev, Dmitry G.
, p. 3301 - 3309 (2016)
Acceptorless dehydrogenative coupling (A...
THERMAL DECOMPOSITION OF PEROXIDE DERIVATIVES OF POLYFLUORINATED β-KETOESTERS
Rakhimov, A. I.,Chapurkin, V. V.,Val'dman, A. I.,Val'dman, D. I.,Saloutin, V. I.,et al.
, (1990)
The thermal flow microcalorimetric metho...
Total oxidation of ethanol over Au/Ce0.5Zr0.5O2 cordierite monolithic catalysts
Topka, Pavel,Klementová, Mariana
, p. 130 - 137 (2016)
The aim of this work was to propose the ...
SPLITTING OF C-C BONDS IN β-DICARBONYL COMPOUNDS CATALYZED BY TRANSITION METAL COMPLEXES
Akhrem, I. S.,Vartanyan, R. S.,Afanas'eva, L. V.,Vol'pin, M. E.
, p. 1217 - 1220 (1983)
-
A dinuclear strontium(II) complex as substrate-selective catalyst of ester cleavage
Cacciapaglia,Di Stefano,Mandolini
, p. 5926 - 5928 (2001)
-
RADICAL REACTION OF ETHYL ORTHOACETATE WITH 3,3,3-TRIFLUOROPROPENE
Khatuntsev, I. I.,Pastushenko, E. V.,Terent'ev, A. B.
, p. 1435 - 1438 (1986)
-
Iodide-induced differential control of metal ion reduction rates: synthesis of terraced palladium-copper nanoparticles with dilute bimetallic surfaces
King, Melissa E.,Personick, Michelle L.
, p. 22179 - 22188 (2018)
Metal nanoparticles possessing a high de...
Kinetics of ethanol dehydrogenation into ethyl acetate
Men'Shchikov,Gol'Dshtein,Semenov
, p. 12 - 17 (2014)
The kinetics of gas-phase dehydrogenatio...
Radical-Induced Reductive Deamination of Amino Acid Esters
Barton, Derek H. R.,Bringmann, Gerhard,Motherwell, Wiliam B.
, p. 68 - 70 (1980)
-
Acetic acid hydrogenation over supported platinum catalysts
Rachmady,Vannice
, p. 322 - 334 (2000)
The kinetic behavior of acetic acid hydr...
Acetic acid hydrogenation to ethanol over supported Pt-Sn catalyst: Effect of Bronsted acidity on product selectivity
Rakshit, Pranab Kumar,Voolapalli, Ravi Kumar,Upadhyayula, Sreedevi
, p. 78 - 90 (2018)
Gas phase hydrogenation of acetic acid w...
Role of the Cu-ZrO2 Interfacial Sites for Conversion of Ethanol to Ethyl Acetate and Synthesis of Methanol from CO2 and H2
Ro, Insoo,Liu, Yifei,Ball, Madelyn R.,Jackson, David H. K.,Chada, Joseph Paul,Sener, Canan,Kuech, Thomas F.,Madon, Rostam J.,Huber, George W.,Dumesic, James A.
, p. 7040 - 7050 (2016)
Well-defined Cu catalysts containing dif...
A green approach to ethyl acetate: Quantitative conversion of ethanol through direct dehydrogenation in a Pd-Ag membrane reactor
Zeng, Gaofeng,Chen, Tao,He, Lipeng,Pinnau, Ingo,Lai, Zhiping,Huang, Kuo-Wei
, p. 15940 - 15943 (2012)
Pincers do the trick: The conversion of ...
-
Hawes,Kabel
, p. 606,609 (1968)
-
-
Connor,Adkins
, p. 3420,3421, 3422 (1932)
-
-
Church,Joshi
, p. 1804 (1951)
-
Catalytic Conversion of Ethanol to n-Butanol Using Ruthenium P-N Ligand Complexes
Wingad, Richard L.,Gates, Paul J.,Street, Steven T. G.,Wass, Duncan F.
, p. 5822 - 5826 (2015)
We report several ruthenium catalysts in...
Selective oxidation of ethanol over Ag, Cu and Au nanoparticles supported on Li2O/Γ-Al2O3
Silbaugh, Trent L.,Devlaminck, Pierre,Sofranko, John A.,Barteau, Mark A.
, p. 40 - 47 (2018)
In an effort to verify a previous striki...
Catalytic Transformation of Ethanol over Microporous Vanadium Silicate Molecular Sieves with MEL Structure (VS-2)
Kannan,Sen,Sivasanker
, p. 304 - 310 (1997)
The transformation of ethanol was carrie...
Structural requirements and reaction pathways in condensation reactions of alcohols on MgyAlOx catalysts
Di Cosimo,Apesteguia,Gines,Iglesia
, p. 261 - 275 (2000)
A study of the effect of composition and...
Production of Pure Aqueous13C-Hyperpolarized Acetate by Heterogeneous Parahydrogen-Induced Polarization
Kovtunov, Kirill V.,Barskiy, Danila A.,Shchepin, Roman V.,Salnikov, Oleg G.,Prosvirin, Igor P.,Bukhtiyarov, Andrey V.,Kovtunova, Larisa M.,Bukhtiyarov, Valerii I.,Koptyug, Igor V.,Chekmenev, Eduard Y.
, p. 16446 - 16449 (2016)
A supported metal catalyst was designed,...
Synthesis of methyl propanoate by Baeyer-Villiger monooxygenases
Van Beek, Hugo L.,Winter, Remko T.,Eastham, Graham R.,Fraaije, Marco W.
, p. 13034 - 13036 (2014)
Methyl propanoate is an important precur...
High-purity alkoxychlorosilanes as new precursors for precipitation of silica
Mirskov,Rakhlin,Adamovich,Voronkov
, p. 194 - 196 (2008)
-
Low-Flammable Parahydrogen-Polarized MRI Contrast Agents
Ariyasingha, Nuwandi M.,Chekmenev, Eduard Y.,Chukanov, Nikita V.,Gelovani, Juri G.,Joalland, Baptiste,Koptyug, Igor V.,Kovtunov, Kirill V.,Nantogma, Shiraz,Salnikov, Oleg G.,Younes, Hassan R.
, p. 2774 - 2781 (2021)
Many MRI contrast agents formed with the...
Synthesis of acetic acid from ethanol-water mixture over Cu/ZnO-ZrO 2-Al2O3 catalyst
Brei, Volodymyr V.,Sharanda, Mykhailo E.,Prudius, Svitlana V.,Bondarenko, Eugenia A.
, p. 196 - 200 (2013)
It was shown that acetic acid can be obt...
Direct synthesis of ethyl acetate from ethanol carried out under pressure
Inui, Kanichiro,Kurabayashi, Toru,Sato, Satoshi
, p. 207 - 215 (2002)
Direct synthesis of ethyl acetate from e...
Revised Mechanisms for Aldehyde Disproportionation and the Related Reactions of the Shvo Catalyst
Gusev, Dmitry G.,Spasyuk, Denis M.
, p. 6851 - 6861 (2018)
It is widely believed that the Shvo cata...
Gas-Phase Acylation Reactions. Substrate and Positional Selectivity of Free Acetylium Ions toward Methylbenzenes
Speranza, Maurizio,Sparapani, Cinzia
, p. 3120 - 3124 (1980)
Free acetylium ions, obtained in the dil...
Deeper Mechanistic Insight into Ru Pincer-Mediated Acceptorless Dehydrogenative Coupling of Alcohols: Exchanges, Intermediates, and Deactivation Species
Nguyen, Duc Hanh,Trivelli, Xavier,Capet, Frédéric,Swesi, Youssef,Favre-Réguillon, Alain,Vanoye, Laurent,Dumeignil, Franck,Gauvin, Régis M.
, p. 4719 - 4734 (2018)
The mechanism of acceptorless dehydrogen...
Copper-mediated decarboxylative coupling of benzamides with potassium malonate monoesters via directed CH cleavage
Takamatsu, Kazutaka,Hirano, Koji,Miura, Masahiro
, p. 450 - 453 (2018)
A copper-mediated decarboxylative coupli...
Impact of the Oxygen Vacancies on Copper Electronic State and Activity of Cu-Based Catalysts in the Hydrogenation of Methyl Acetate to Ethanol
Xi, Yushan,Wang, Yue,Yao, Dawei,Li, Antai,Zhang, Jingyu,Zhao, Yujun,Lv, Jing,Ma, Xinbin
, (2019)
Reducible oxides supported copper-based ...
ALKANEPERSULFONIC ACIDS AS NEW OXIDIZING AGENTS IN THE BAYER-VILLIGER REACTION
Safiullin, R. L.,Volgarev, A. N.,Komissarov, V. D.,Tolstikov, G. A.
, p. 1998 (1990)
-
RADICAL TELOMERIZATION OF 3,3,3-TRIFLUOROPROPENE WITH 2-METHYL-1,3-DIOXOLANE
Terent'ev, A.B.,Pastushenko, E.V.,Kruglov, D.E.,Ryabinina, T.A.
, p. 2197 - 2200 (1992)
The telomerization of 3,3,3-trifluoropro...
FeSBA-15-supported ruthenium catalyst for the selective hydrogenolysis of carboxylic acids to alcoholic chemicals
Li, Wenjing,Ye, Linmin,Chen, Jin,Duan, Xinping,Lin, Haiqiang,Yuan, Youzhu
, p. 53 - 59 (2015)
Ordered mesoporous FeSBA-15-supported Ru...
Effect of Support in Ethanol Oxidation on Molybdenum Oxide
Zhang, Weimin,Desikan Anantha,Oyama, S. Ted
, p. 14468 - 14476 (1995)
The oxidation of ethanol on MoO3 support...
Bifunctional mesoporous organic-inorganic hybrid silica for combined one-step hydrogenation/esterification
Tang, Yang,Miao, Shaojun,Shanks, Brent H.,Zheng, Xiaoming
, p. 310 - 317 (2010)
Bifunctional mesoporous organic-inorgani...
Facile synthesis of homogeneous CsxWO3 nanorods with excellent low-emissivity and NIR shielding property by a water controlled-release process
Guo, Chongshen,Yin, Shu,Yan, Mei,Sato, Tsugio
, p. 5099 - 5105 (2011)
A systematic investigation of the synthe...
Esterase activities of Brevibacterium sp. R312 and Brevibacterium linens 62
Lambrechts,Galzy
, p. 1464 - 1471 (1995)
-
Highly selective 1-butanol obtained from ethanol catalyzed by mixed metal oxides: Reaction optimization and catalyst structure behavior
Rechi Siqueira, Marcos,Micali Perrone,Metzker, Gustavo,de Oliveira Lisboa, Daniela Correa,Thoméo, Jo?o Cláudio,Boscolo, Maurício
, (2019)
Synthesis and characterization of a copp...
Structural investigation of solid-acid-promoted Pd/SDB catalysts for ethyl acetate production from ethanol
Lee,Zheng,Chang
, p. 3400 - 3404 (2001)
Catalyzed by styrene-divinylbenzene copo...
-
Ruggli et al.
, p. 411,413 (1939)
-
-
Kagan,Ssobolew,Ljubarski
, p. 1142 (1935)
-
Thermal decomposition of acetyl propionyl peroxide in acetone-d6
Skakovskii,Stankevich,Tychinskaya,Shirokii,Choban,Murashko,Rykov
, p. 1719 - 1725 (2004)
The kinetics of thermolysis of acetyl pr...
Facile Preparation of Methyl Phenols from Ethanol over Lamellar Ce(OH)SO4· xH2O
Guo, Jinqiu,Feng, Zongjing,Xu, Jun,Zhu, Jie,Zhang, Guanghui,Du, Yaping,Zhang, Hongbo,Yan, Chunhua
, p. 6162 - 6174 (2021)
Ethanol transformation with high product...
The Reaction of with Triethoxysilane in the Presence of PPh3: a New Method for Synthesis of
Marciniec, Bogdan,Maciejewski, Hieronim,Gulinski, Jacek
, p. 717 - 718 (1995)
The reaction of with triethoxysilane in...
Removal of the copper catalyst from atom transfer radical polymerization mixtures by chemical reduction with zinc powder
Canturk, Fatma,Karagoz, Bunyamin,Bicak, Niyazi
, p. 3536 - 3542 (2011)
Simple mixing of an atom transfer radica...
DEUTERIUM ISOTOPE EFFECTS IN THE THERMAL DECOMPOSITION OF β-HYDROXY KETONES AND β-HYDROXY ESTERS
Quijano, J.,Rodriguez, M. M.,Yepes, M. del S.,Gallego, L.H.
, p. 3555 - 3558 (1987)
Small primary and cumulative secondary i...
Insight into the balancing effect of active Cu species for hydrogenation of carbon-oxygen bonds
Wang, Yue,Shen, Yongli,Zhao, Yujun,Lv, Jing,Wang, Shengping,Ma, Xinbin
, p. 6200 - 6208 (2015)
Hydrogenation of carbon-oxygen (C-O) bon...
Dehydrogenative ester synthesis from enol ethers and water with a ruthenium complex catalyzing two reactions in synergy
Ben-David, Yehoshoa,Diskin-Posner, Yael,Kar, Sayan,Luo, Jie,Milstein, David,Rauch, Michael
supporting information, p. 1481 - 1487 (2022/03/07)
We report the dehydrogenative synthesis ...
Chemoselective Hydrogenation of Olefins Using a Nanostructured Nickel Catalyst
Klarner, Mara,Bieger, Sandra,Drechsler, Markus,Kempe, Rhett
supporting information, p. 2157 - 2161 (2021/05/21)
The selective hydrogenation of functiona...
Background-Free Proton NMR Spectroscopy with Radiofrequency Amplification by Stimulated Emission Radiation
Appelt, Stephan,Chekmenev, Eduard Y.,Joalland, Baptiste,Theis, Thomas
, p. 26298 - 26302 (2021/10/04)
We report on the utility of Radiofrequen...
141-78-6 Process route
-
-
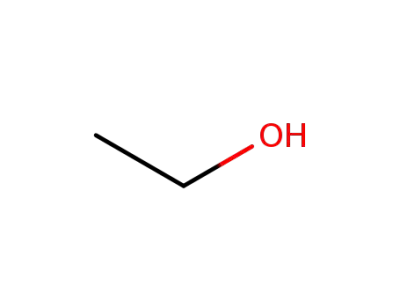
64-17-5
ethanol

-
-
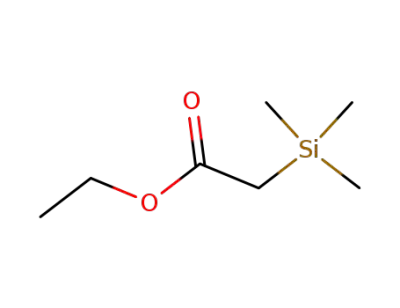
4071-88-9
trimethylsilanyl-acetic acid ethyl ester

-
-
1825-62-3
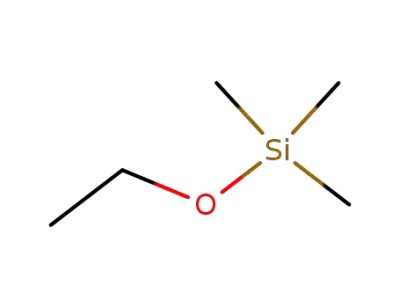
ethyl trimethylsilyl ether

-
-
141-78-6
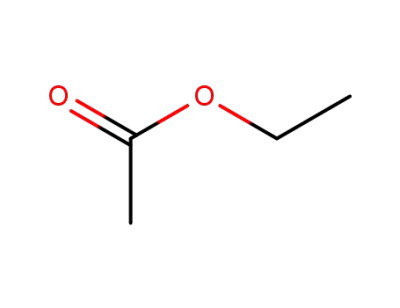
ethyl acetate
| Conditions | Yield |
|---|---|
|
|
|
|
In
ethanol;
boiling of (CH3)3Si(CH2COOC2H5) in abs. ethanol for a longer period of time;;
|
-
-
105-36-2
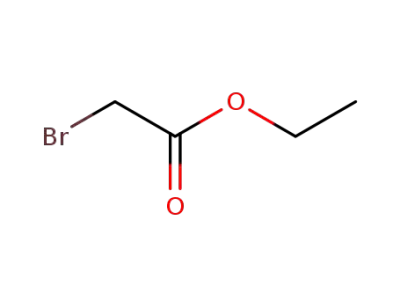
ethyl bromoacetate

-
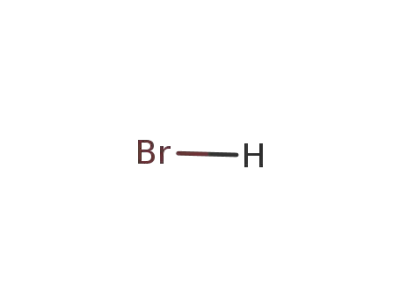
-
10035-10-6,12258-64-9
hydrogen bromide

-
-
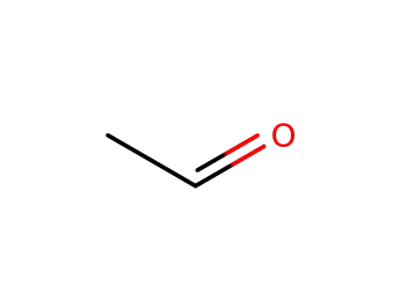
75-07-0,9002-91-9
acetaldehyde

-

-
141-78-6
ethyl acetate
| Conditions | Yield |
|---|---|
|
at 250 - 300 ℃;
|
141-78-6 Upstream products
-
110-86-1
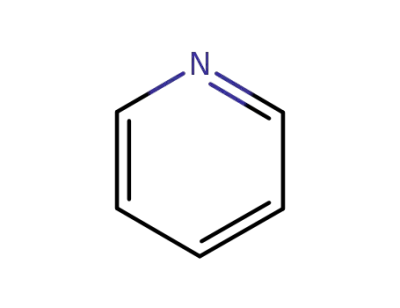
pyridine
-
128-09-6
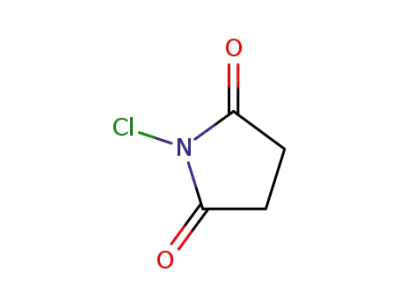
N-chloro-succinimide
-
64-17-5

ethanol
-
71-43-2
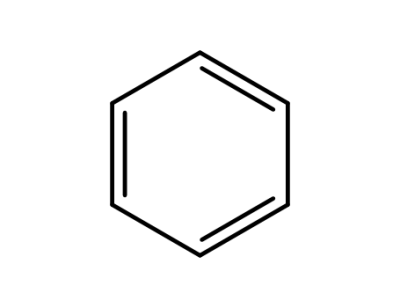
benzene
141-78-6 Downstream products
-
7335-06-0
1-ethylpyrrolidine
-
1530-87-6
1-piperidinylcarbonnitrile
-
825-25-2
2-cyclopentylidenecyclopentan-1-one
-
618-42-8
1-piperidin-1-yl-ethanone
Relevant Products
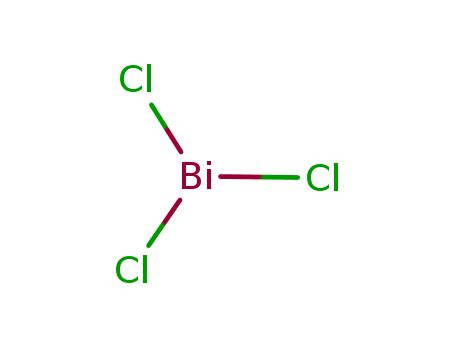
-
Bismuth Octoate
CAS:67874-71-9
-
n-Butyl Acetate
CAS:123-86-4
-
2-fluorobenzonitrile
CAS:394-47-8