7787-60-2
- Product Name:Bismuth Trichloride
- Molecular Formula:BiCl3
- Purity:99%
- Molecular Weight:315.339
Product Details
Manufacturers supply cost-effective and customizable Bismuth Trichloride 7787-60-2
- Molecular Formula:BiCl3
- Molecular Weight:315.339
- Appearance/Colour:White crystal and crystalline powder
- Melting Point:230-232 °C(lit.)
- Boiling Point:447oC
- Flash Point:430°C
- PSA:0.00000
- Density:4.75 g/cm3
- LogP:2.06850
Bismuth(III) chloride(Cas 7787-60-2) Usage
|
General Description |
Bismuth(III) chloride, also known as bismuth trichloride, is a chemical compound with the formula BiCl3. It is a yellow, hygroscopic solid that is soluble in water and ethanol. Bismuth(III) chloride is commonly used as a catalyst in the production of organic chemicals, as well as in the synthesis of pharmaceuticals and other compounds. It is also used in the production of bismuth oxychloride, a pearlescent pigment used in cosmetics. Bismuth(III) chloride has also been studied for its potential applications in medical imaging and as a potential treatment for certain types of cancer. However, it is important to handle bismuth(III) chloride with caution, as it is toxic and can cause irritation to the skin, eyes, and respiratory system. |
InChI:InChI=1/Bi.3ClH.3H/h;3*1H;;;/q+3;;;;;;/p-3/rBiH3.3ClH/h1H3;3*1H/q+3;;;/p-3
7787-60-2 Relevant articles
The pseudobinary systems Bi2Ch3-Bix3 and the ternary phases on their boundary lines (Ch = S, Se, Te; X = Cl, Br, I), I: Bismuth sulfide halides
Oppermann, Heinrich,Petasch, Uwe
, p. 725 - 740 (2003)
The phase diagrams of the systems Bi2S3-...
New routes to transition metal-carbido species: Synthesis and characterization of the carbon-centered trigonal prismatic clusters [W 6CCl18]n- (n = 1, 2, 3)
Welch, Eric J.,Crawford, Nathan R. M.,Bergman, Robert G.,Long, Jeffrey R.
, p. 11464 - 11465 (2003)
Simultaneous reduction of WCl6 and CCl4 ...
In-situ study of the solid-gas reaction of biCl3 to biOCl via the intermediate hydrate biCl3·H2O
Wosylus, Aron,Hoffmann, Stefan,Schmidt, Marcus,Ruck, Michael
, p. 1469 - 1471 (2010)
At ambient conditions the hydrolysis of ...
Constitutional isomerism of BiW6Cl15: (BiCl)[W 6Cl14] and (BiCl2)[W6Cl 13]
Stroebele, Markus,Meyer, H.-Juergen
, p. 1517 - 1519 (2009)
The compound (BiCl)[W6Cl14] was previous...
Reactions of Chlorine with Liquid Metals. 3. Bismuth
Balooch, M.,Siekhaus, W. J.,Olander, D. R.
, p. 1671 - 1676 (1986)
The reaction of molecular chlorine with ...
Investigations on the pseudobinary system Bi2Se3/BiCl3
Petasch,Oppermann
, p. 169 - 173 (1997)
The phase diagram of the pseudobinary sy...
Syntheses, properties and crystal structures of the cluster salts Bi 6[PtBi6Cl12] and Bi2/3[PtBi 6Cl12]
Hampel, Silke,Ruck, Michael
, p. 1150 - 1156 (2006)
Melting reactions of Bi with Pt and BiCl...
Solution-phase template approach for the synthesis of Cu2S nanoribbons
Li, Zhengquan,Yang, Huan,Ding, Yue,Xiong, Yujie,Xie, Yi
, p. 149 - 151 (2006)
In this paper, we have developed a solut...
Synthesis, structure and properties of the first hybrid Bi 2Cl93- supramolecular compound based on macrocyclic rotator-stator assembly
Peng, Yan-Hong,Sun, Shao-Fa
, p. 29 - 32 (2012)
The first hybrid Bi(III) supramolecular ...
Facile reduction of early transition metal halides with nonconventional, mild reductants (see abstract)
Hay, Daniel N.T.,Adams, Juan A.,Carpenter, Jason,DeVries, Stephanie L.,Domyancich, John,Dumser, Bruce,Goldsmith, Shawn,Kruse, Melissa A.,Leone, Angela,Moussavi-Harami, Farid,O'Brien, Jennifer A.,Pfaffly, Jennifer R.,Sylves, Michael,Taravati, Parisa,Thomas, Jacob L.,Tiernan, Breck,Messerle, Louis
, p. 644 - 648 (2004)
Reduction of MoCl5 with Bi in a sealed b...
The bicyclic polyselenium cation Se102+ in the structure of Se10[Bi4Cl14]
Beck, Johannes,Eck, Steffen J.
, p. 1910 - 1912 (2011/01/05)
In the ternary system Se/Bi/Cl a new pol...
Crystalline and glassy phases in the ternary system Tl/Bi/Cl: Synthesis and crystal structures of the thallium(I) chloridobismutates(III) Tl3BiCl 6 and TlBi2Cl7
Beck, Johannes,Benz, Sebastian
, p. 928 - 935 (2010/09/10)
Slow cooling of melts composed of TlCl a...
7787-60-2 Process route
-

-
7440-69-9
bismuth

-
-
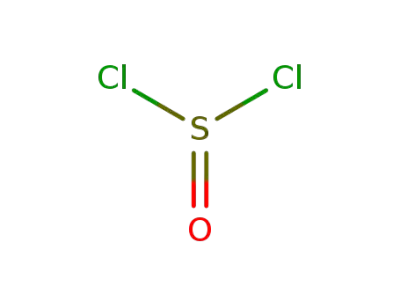
7719-09-7
thionyl chloride

-
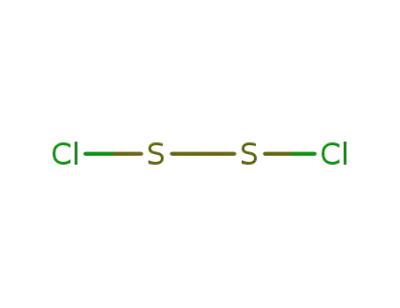
-
10025-67-9
disulfur dichloride

-
-
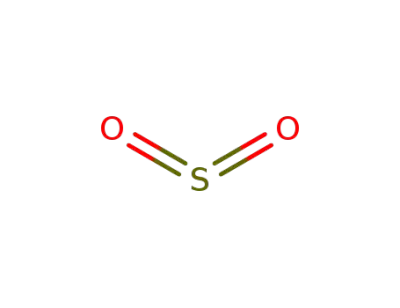
7446-09-5,12143-17-8,89125-89-3
sulfur dioxide

-
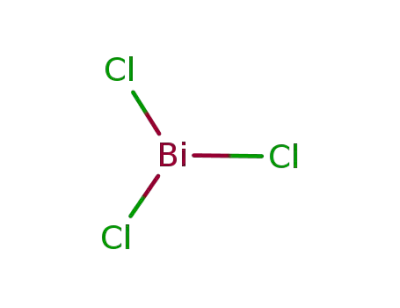
-
7787-60-2
bismuth(III) chloride
| Conditions | Yield |
|---|---|
|
reaction at 150-200 °C;;
|
|
|
reaction at 150-200 °C;;
|
-

-
7440-69-9
bismuth

-
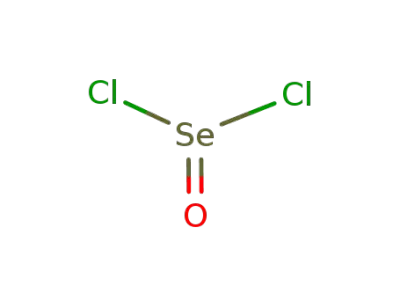
-
7791-23-3
selenium oxychloride

-

-
7787-60-2
bismuth(III) chloride

-
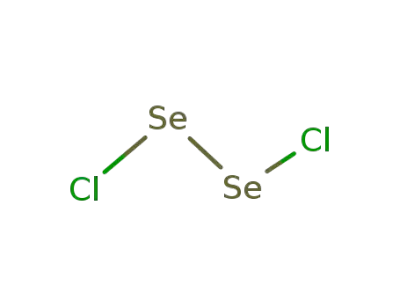
-
10025-68-0
diselenium dichloride
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
Bi is soluble in excess of SeOCl2 with formation of BiCl3 and Se2Cl2;;
|
|
|
In
neat (no solvent);
Bi is soluble in excess of SeOCl2 with formation of BiCl3 and Se2Cl2;;
|
7787-60-2 Upstream products
-
593-81-7
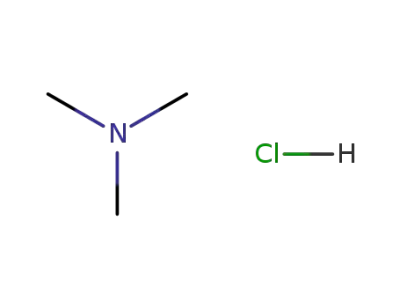
trimethylamine hydrochloride
-
603-33-8
triphenylbismuthane
-
75-44-5
phosgene
-
7647-01-0
hydrogenchloride
7787-60-2 Downstream products
-
312629-10-0
tris(3-benzyloxyphenyl)bismuthane
-
7440-69-9

bismuth
-
7647-01-0

hydrogenchloride
-
994-31-0
chlorotriethylstannane
Relevant Products
-
Bismuth Octoate
CAS:67874-71-9
-
1,8-Dibromooctane
CAS:4549-32-0
-
Bismuth Hydroxide
CAS:10361-43-0