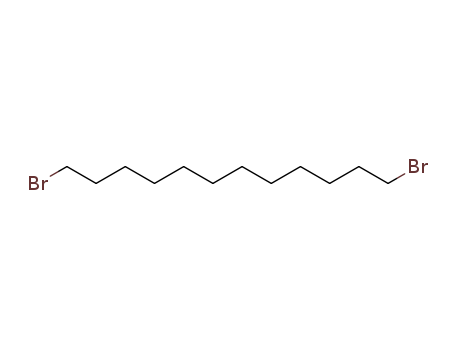
4549-32-0
- Product Name:1,8-Dibromooctane
- Molecular Formula:C8H16Br2
- Purity:99%
- Molecular Weight:272.023
Product Details
Top quality factory supply 4549-32-0 1,8-Dibromooctane at low price
- Molecular Formula:C8H16Br2
- Molecular Weight:272.023
- Appearance/Colour:colourless to pale yellow liquid
- Vapor Pressure:0.011mmHg at 25°C
- Melting Point:12-16 °C(lit.)
- Refractive Index:n20/D 1.498(lit.)
- Boiling Point:271 °C at 760 mmHg
- Flash Point:154.9 °C
- PSA:0.00000
- Density:1.455 g/cm3
- LogP:4.11680
1,8-Dibromooctane(Cas 4549-32-0) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 30, p. 780, 1965 DOI: 10.1021/jo01014a031 |
|
Synthesis |
1,8-Dibromooctane was prepared by the reaction of cyclooctene with sulfuric acid and hydrobromination under the catalyst. |
|
Application |
1,8-Dibromooctane is used as a reagent in the synthesis of novel 7-aminoalkyl-substituted flavonoid derivatives as potential cholinesterase inhibitors and 3,3’-(Octane-1,8-diyl)bis(1-ethyl-imidazolium) bromide. |
InChI:InChI=1/C8H16Br2/c9-7-5-3-1-2-4-6-8-10/h1-8H2
4549-32-0 Relevant articles
Process intensification-assisted conversion of α,ω-alkanediols to dibromides
Mekala, Shekar,Hahn, Roger C.
supporting information, p. 630 - 632 (2015/03/03)
The increasingly widespread applications...
First syntheses of model long-chain trichloro[ω-(trimethylsilyl)alkynyl]silanes suitable for self-assembled monolayers on silicon surfaces
Banaszak, Estelle,Xu, Li-Wen,Bardeau, Jean-Fran?ois,Castanet, Anne-Sophie,Mortier, Jacques
body text, p. 3961 - 3966 (2009/09/30)
The preparation of the title compounds i...
ALLYL SULFIDE COMPOUNDS, AND COMPOSITIONS AND METHODS USING SAID COMPOUNDS FOR REPELLING BLOOD-FEEDING ARTHROPODS
-
Page/Page column 15, (2008/12/04)
This invention relates to compositions o...
Ionic liquids as reagents and solvents in conjunction with microwave heating: Rapid synthesis of alkyl halides from alcohols and nitriles from aryl halides
Leadbeater, Nicholas E.,Torenius, Hanna M.,Tye, Heather
, p. 2253 - 2258 (2007/10/03)
We show that using ionic liquids as reag...
4549-32-0 Process route
-
-
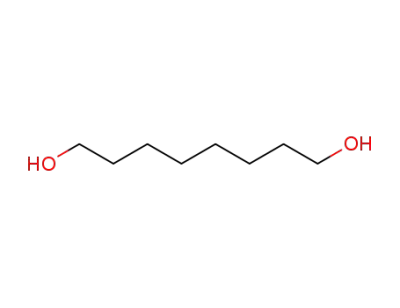
629-41-4
1,8-Octanediol

-
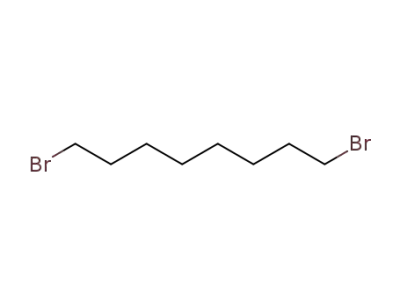
-
4549-32-0
1,8-dibromooctane

-
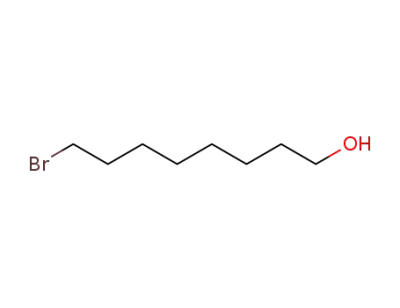
-
50816-19-8
8-bromooctanol
| Conditions | Yield |
|---|---|
|
With
hydrogen bromide;
In
water; toluene;
Reflux;
|
90% |
|
With
hydrogen bromide;
In
pyridine;
for 72h;
Further Variations:;
Solvents;
reaction times;
Product distribution;
Heating;
|
87% |
|
With
hydrogen bromide;
In
toluene;
Heating;
|
85% 9% |
|
With
hydrogen bromide;
In
toluene;
Heating;
|
85% 9% |
|
With
hydrogen bromide;
at 60 ℃;
for 96h;
|
69% |
|
With
hydrogen bromide;
In
para-xylene; water;
at 130 ℃;
for 11h;
Dean-Stark;
|
|
|
With
hydrogen bromide;
In
n-heptane; water;
at 20 - 55 ℃;
Product distribution / selectivity;
|
-

-
629-41-4
1,8-Octanediol

-
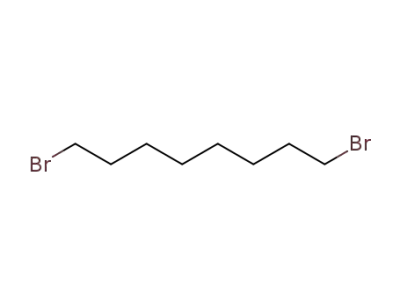
-
4549-32-0
1,8-dibromooctane
| Conditions | Yield |
|---|---|
|
With
toluene-4-sulfonic acid; 1-(1-methylethyl)-3-methylimidazolium bromide;
In
toluene;
at 200 ℃;
for 0.05h;
under 10343 Torr;
microwave irradiation;
|
86% |
|
With
hydrogen bromide;
at 90 - 140 ℃;
|
|
|
In
diethyl ether; water;
|
82 mol % |
4549-32-0 Upstream products
-
629-41-4

1,8-Octanediol
-
51306-09-3
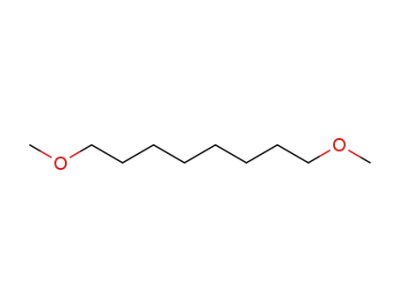
1,8-dimethoxyoctane
-
32038-98-5
N,N'-Octamethylenedibenzamide
-
110042-34-7
1,8-bis(p-anisyloxy)octane
4549-32-0 Downstream products
-
296-30-0
1,10-Diaza-cyclooctadecan
-
120808-65-3
1,10-bis(p-tolylsulphonyl)-1,10-diazacyclo-octadecane
-
94680-04-3
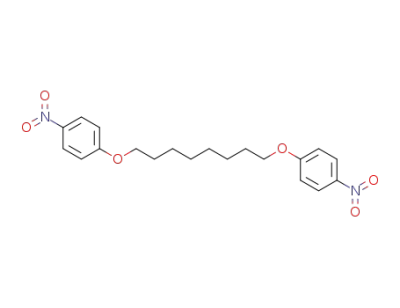
1,8-bis(4-nitrophenoxy)octane
-
110662-67-4
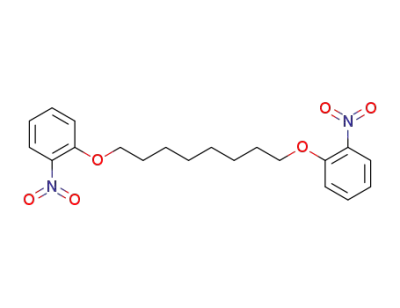
1,8-bis-(2-nitro-phenoxy)-octane
Relevant Products
-
3-Bromobenzanthrone
CAS:81-96-9
-
1,12-Dibromododecane
CAS:3344-70-5
-
Bismuth Trichloride
CAS:7787-60-2