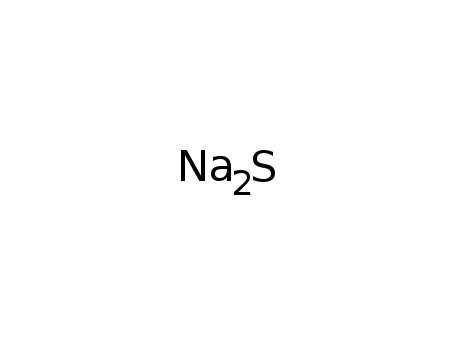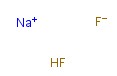
1333-83-1
- Product Name:Sodium hydrogen fluoride
- Molecular Formula:NaHF2
- Purity:99%
- Molecular Weight:61.994516 [g/mol]
Product Details
Cost-effective and customizable Sodium hydrogen fluoride 1333-83-1 in stock
- Molecular Formula:NaHF2
- Molecular Weight:61.994516 [g/mol]
- Appearance/Colour:white crystalline solid
- Vapor Pressure:0.01Pa at 20℃
- Melting Point:>68 °C (dec.)
- Boiling Point:19.5 °C at 760 mmHg
- PSA:0.00000
- Density:2.08 g/cm3
- LogP:-2.46330
Sodium hydrogen difluoride(Cas 1333-83-1) Usage
|
Air & Water Reactions |
Water soluble. Reacts with water liberating heat and forming a corrosive solution. The reaction is not violent. |
|
Reactivity Profile |
Acidic salts, such as SODIUM BIFLUORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. |
|
Hazard |
Highly toxic, strong irritant to tissue. |
|
Health Hazard |
INHALATION OF DUST: Irritating and possibly corrosive to mucous membranes. EYES: Irritating. INGESTION: Salty or soapy taste, salivation, nausea, burning or crampy abdominal pain, vomiting, diarrhea, muscle weakness, tremors. Rare: transient epileptiform convulsions, followed by CNS depression. Shock. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
This material is very toxic to humans by ingestion; between 1 teaspoonful and 1 ounce may be fatal. Inhalation of dust may cause irritation to respiratory tract. Skin contact may result in irritation and ulceration; eye contact may cause burns. To fight fire, use water, foam, CO2, dry chemicals. When heated to decomposition it emits toxic fumes of Fand Na2O. See also FLUORIDES and HYDROFLUORIC ACID. |
|
General Description |
Sodium bifluoride is a white crystalline solid. Sodium hydrogen difluoride is soluble in water. Sodium hydrogen difluoride is corrosive to tissue. Sodium hydrogen difluoride is used as a preservative for anatomical and zoological specimens, in metal plating and for many other uses. |
InChI:InChI=1/FH.Na/h1H;/q;+1
Relevant Products
-
Sodium sulfide
CAS:1313-82-2
-
Potassium hydrogen fluoride
CAS:7789-29-9
-
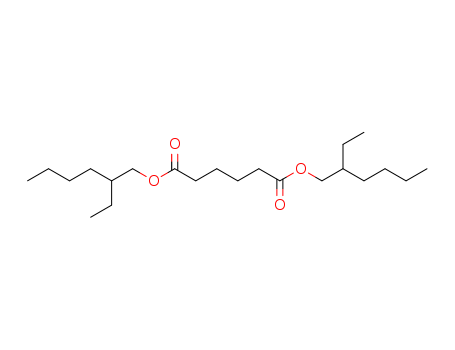
Dioctyl Adipate
CAS:103-23-1