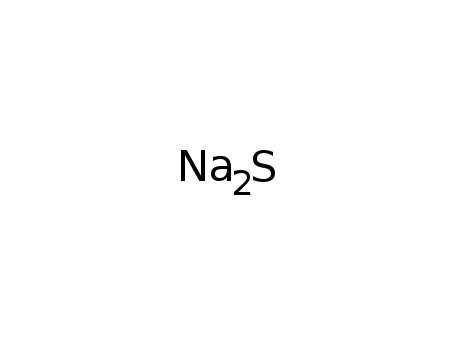7789-29-9
- Product Name:Potassium hydrogen fluoride
- Molecular Formula:F2HK
- Purity:99%
- Molecular Weight:78.11
Product Details
Cost-effective and customizable Potassium hydrogen fluoride 7789-29-9 factory
- Molecular Formula:F2HK
- Molecular Weight:78.11
- Appearance/Colour:A white, odorless crystalline powder
- Melting Point:239 °C
- Boiling Point:19.5 °C at 760 mmHg
- PSA:0.00000
- Density:2.37 g/mL at 25 °C(lit.)
- LogP:-2.46330
Potassium hydrogen fluoride(Cas 7789-29-9) Usage
|
Air & Water Reactions |
Water soluble giving strongly acidic solutions. |
|
Reactivity Profile |
Acidic salts, such as Potassium hydrogen fluoride, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. |
|
Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
Safety Profile |
A poison. Very corrosive and reactive. A corrosive irritant to the eyes, skin, and mucous membranes. When heated to decomposition it emits toxic fumes of F and K2O. See also FLUORIDES and HYDROFLUORIC ACID. |
|
Purification Methods |
It crystallises from water. It is very soluble in hot H2O and 41% at 21o. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 237 1963.] |
|
General Description |
Colorless crystals, corrosive to tissues, etches glass. |
InChI:InChI=1/FH.K/h1H;/q;+1
Relevant Products
-
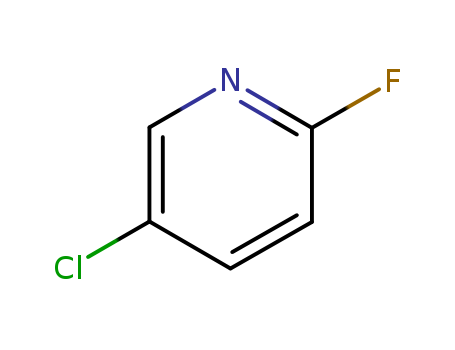
5-Chloro-2-fluoropyridine
CAS:1480-65-5
-
Sodium sulfide
CAS:1313-82-2
-
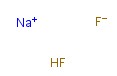
Sodium hydrogen fluoride
CAS:1333-83-1