1313-82-2
- Product Name:Sodium sulfide
- Molecular Formula:Na2S
- Purity:99%
- Molecular Weight:78.0455
Product Details
Cost-effective and customizable Sodium sulfide 1313-82-2 supplier
- Molecular Formula:Na2S
- Molecular Weight:78.0455
- Appearance/Colour:crystals of varied colour, with a repulsive odour
- Melting Point:950 ºC
- Boiling Point:174oC
- PSA:25.30000
- Density:1.86 g/cm3
- LogP:-0.53540
Sodium sulfide(Cas 1313-82-2) Usage
|
Sodium sulfide |
Sodium sulfide is also known as smelly soda and stinky base. At room temperature, the pure product is colorless or slightly purple prismatic crystal. The industrial sodium sulfide often exhibits pink, reddish brown or yellowish brown color for containing impurities. It has rotten egg smell and is corrosive and toxic. Its density is 2.427. It will be subject to decomposition at 920 ℃. It is soluble in cool water and easily soluble in hot water with dissolving in water almost fully being hydrolyzed into sodium hydroxide and sodium hydrosulfide (at 10 ℃, the solubility is 15.4; while the solubility is 57.2 g at 90 ℃). The aqueous water exhibits strongly alkalinity and is corrosive on copper, wood, skin, etc. It is slightly soluble in ethanol but insoluble in ether. When being encountered strong acid, the sodium sulfide will release hydrogen sulfide. It will be subject to deliquescence in air and is easily oxidized into sodium thiosulfate. Sodium sulfide is mainly used as the raw materials of hides depilatories, pulp cooking agent, and sulfur dye, the reducing agents of dye intermediates, fabric dyeing mordant, and ore flotation agent. It can also be used as viscose fiber desulfurizer and the raw material for production of sodium hydrosulfide and sodium polysulfide. The sodium sulfide in our county was originated in the 1830s. The production of it was earliest started from a chemical plant in Dalian, Liaoning in small-scale. Upon entering into the mid-1980s to the 1990s, with the vigorous development of the international chemical industry, the domestic industry had undergone a fundamental transformation with both the number and scale of production being dramatically increased with rapid development. The production area of sodium sulfide centered in Shanxi Yuncheng has quickly expanded to a dozen of other provinces or cities including Yunnan, Xinjiang, Inner Mongolia, Gansu, Qinghai, Ningxia, and Shaanxi. The national annual production capacity has increased from the value of 420,000 tons by the end of the 80s to a value of 640,000 tons in mid-1990s. The region with the fastest growing includes Inner Mongolia, Gansu, and Xinjiang region at northwest of China. The production capacity has reached 200,000 tons in Inner Mongolia, which has become the largest production base of sodium sulfide in China. Figure 1 is a picture of yellow flaky sodium sulfide The above information is edited by the lookchem of Dai Xiongfeng. |
|
Industrial sodium sulfide |
Industrial sodium sulfide is generally a mixture with different numbers of crystalline water; the molecular formula is Na2S ? nH2O; it exhibits as yellow or reddish-brown massive, flaky and granular and is mainly used in paper, dyes, mineral processing, printing and dyeing industries. GB/T 10500-2000 standard has classified the industrial sodium sulfide products into three categories: Category 1 is ordinary sodium sulfide (commonly known as red base); Class 2 is low-iron sodium sulfide (commonly known as the yellow base); Class 3 is sodium sulfide of high content. Figure 2 the reference quality indicators of industrial sodium sulfide Packing, storage and shipping: sodium sulfide belongs to alkaline corrosive substances, classification: GB 8.2 class, number:82011. It is packed with a tight leakproof iron drums with the net weight per barrel being 25.50 kg or 100 kg. The packaging should contain obvious "drugs" and "corrosive substance" signs. It should be stored in a dry, airy shed asbestos with the container must be intact. It can’t be stored and shipped together with acidic materials and oxidizing agents. |
|
Toxicity |
Sodium sulfide is strongly corrosive to the skin. Worker subjecting to contact with a solution of sodium sulfide has their hand skin get ruffling and redness. During the operation, you should note that: upon inadvertently contact with skin, you should rinse with water. After the sodium sulfide droplets or small pieces falling into eyes, immediately wash with water for 15 min and send to hospital for treatment. To protect the skin, it is recommended to wash hands with a weak acetic acid solution and then coated with oily ointment. Pay attention to the protection of eye. |
|
Preparation of polyarylene sulfide |
We can take industrial sodium sulfide and poly-halogenated aromatic compounds as raw materials; apply multi-component composite catalyst or additive and carry out segmented poly-condensation at normal pressure in high-boiling polar organic solvent (such as hempa) for generating linear high molecular weight polyarylene sulfide. The reaction conversion rate is high with the product being white granular and with excellent mechanical properties, thermal properties and thermal processing stability. Additionally supplement of a certain amount of cross-linking agent can generate higher molecular weight branched or cross-linked polyarylene sulfide. |
|
Production method |
Pulverized coal reduction method: put mirabilite and coal powder in a mixing ratio of 100: (21 to 22.5) (weight ratio) for calcination and reduction at 800~1100 ℃. The resultant after cooling is molten into a liquid. After standing for clarification, the upper portion of the alkaline solution was concentrated to obtain a solid sulfide. The flake (or granules)-like sodium sulfide is made through the transition tank and flaking. The reaction equation is as below: Na2SO4 + 2C → Na2S + 2CO2 Absorption method: use 380~420 g/L sodium hydroxide solution to absorb H2S> 85% containing hydrogen sulfide waste gas; the resulting product was concentrated by evaporation to obtain sodium sulfide products. Its reaction equation is as below: H2S + 2NaOH → Na2S + 2H2O Barium sulfide method: use sodium sulfide and barium sulfide for cross-metathesis reaction to get precipitated barium sulfate. During this process, we can get the byproduct sodium sulfide. The reaction formula is as below: BaS + Na2SO4 → Na2S + BaSO4 ↓ Gas reduction method: in the presence of iron catalyst, put hydrogen (or carbon monoxide, coal gas, methane gas) into boiling furnace for reaction with sodium sulfate, we can obtain high-quality anhydrous granular sodium sulfide (containing Na2S: 95%~97% ). Its reaction equation is: Na2SO4 + 4CO → Na2S + 4CO2 Na2SO4 + 4H2 → Na2S + 4H2O Refined method using the byproduct (4% of sodium sulfide) during the production of precipitated barium sulfate as raw material, pump it into dual-effect evaporator for concentration into 23%; it further enters into the mixing tank for removing iron as well as carbon; pump it into the evaporator (manufactured by pure nickel material) to evaporate the lye into a certain concentration and further send it into the roller squeezing apparatus for flaking and further obtain the finished product after screening and packaging. Pulverized coal reduction method is the traditional production method for making sodium sulfide. During the manufacturing process, improve the equipment and materials and increase the iron removal process so that the products can meet standards. |
|
Toxicity grading |
highly toxic |
|
Acute toxicity |
oral-rat LD50: 208 mg/kg; Oral-Mouse LD50: 205 mg/kg |
|
Hazardous characteristics of explosive |
it is explosive upon heating and collision. |
|
Flammability and hazard characteristics |
it release toxic hydrogen sulfide gas upon acids; anhydrous sodium sulfide is flammable; heating can release toxic fumes of sulfur oxides |
|
Storage characteristics |
Treasury: ventilation, low-temperature and dry; store it separately from oxidants and acids |
|
Extinguishing agent |
water, sand |
|
Preparation |
Sodium sulfide is prepared by heating sodium bisulfate with sodium chloride and coal above 950°C. The product mixture is extracted with water and the hydrated sulfide is obtained from the solution by crystallization: NaHSO4 + NaCl + 2C → Na2S + 2CO2↑ + HCl↑Sodium sulfide also is produced from its elements in liquid ammonia: Na + 2S → Na2S. |
|
Air & Water Reactions |
Aqueous solutions of sodium sulfide when exposed to air slowly convert to sodium hydroxide and sodium thiosulfate. The crystalline form upon exposure to air forms hydrogen sulfide and sodium carbonate [Merck 11th ed. 1989]. |
|
Reactivity Profile |
SODIUM SULFIDE is a white to yellow crystalline material, flammable. Can explode on rapid heating or when shocked. Violent reaction with carbon, charcoal, diazonium salts, N,N-dichloromethylamine, strong oxidizers, water. On contact with acids Sodium sulfide liberates highly toxic and flammable hydrogen sulfide gas. When heated to decomposition Sodium sulfide emits toxic fumes of sodium oxide, and oxides of sulfur [Bretherick, 5th ed., 1995, p. 1729]. |
|
Hazard |
Flammable, dangerous fire and explosion risk. Strong irritant to skin and tissue, liberates toxic hydrogen sulfide on contact with acids. |
|
Health Hazard |
Caustic action on skin and eyes. If ingested may liberate hydrogen sulfide in stomach. |
|
Fire Hazard |
Special Hazards of Combustion Products: Irritating sulfur dioxide is produced in fire. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
A poison by ingestion and intraperitoneal routes. Flammable when exposed to heat or flame. Unstable and can explode on rapid heating or percussion. Reacts violently with carbon, diazonium salts, n,n-dichloromethylamine, onitroaniline diazonium salt, water. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFIDES |
|
Purification Methods |
Some purification of the hydrated salt can be achieved by selecting large crystals and removing the surface layer (contaminated with oxidation products) by washing with distilled water. Other metal ions can be removed from Na2S solutions by passage through a column of Dowex ion-exchange A-1 resin, Na+-form. The hydrated salt can be rendered anhydrous by heating it in a stream of H2 or N2 until water is no longer evolved. (The resulting cake should not be heated to fusion because it is readily oxidised.) Recrystallise it from distilled water [Anderson & Azowlay J Chem Soc, Dalton Trans 469 1986]. Note that sodium sulfide hydrolyses in H2O to form NaHS + H2O, and is therefore alkaline. A 0.1N solution in H2O is 86% hydrolysed at room temperature. Its solubility in H2O is 8% at 0o, 12% at 20o and 30% at 50o. The anhydrous salt is obtained by allowing it to stand in a vacuum over conc H2SO4 or P2O5 at 45o to start with, then at 30-35o when the salt contains 4% of water. The last traces of water are removed by heating to 700o in a glass or porcelain tube in a stream of H2 to give pure H2S. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 358-360 1963.] |
|
Synthesis and Application in Silver Nanocube Production |
Addition of trace amounts of Na2S or NaHS drastically improves the production rate of silver nanocubes. Sulfide species interact strongly with silver, facilitating the formation of Ag2S nanoparticles. |
|
Utilization in NOx Reduction |
Na2S can effectively reduce NO2 to N2, converting it into nontoxic sodium sulfate (Na2SO4). Also capable of removing SO2 effectively, enabling simultaneous treatment of NOx and SO2 in exhaust gases. |
|
Chemical Reactions and Applications |
Reacts rapidly with di(1-alkynyl) sulfoxides and sulfones to form six-membered heterocyclic compounds. Used in the formation of novel heterocyclic systems. |
|
Industrial and Chemical Applications |
Used in various industries such as pulp and paper, water treatment, textile, and chemical manufacturing. |
|
Biological Effects and Medical Applications |
Na2S and hydrogen sulfide (H2S) have been studied for their anti-inflammatory and anti-apoptotic effects in some studies. |
|
Physical properties |
White cubic crystal; hygroscopic; density 1.856 g/cm3; melts at 1,172°C; soluble in water 18.6 g/100mL at 20°C and 39 g/100mL at 50°C; aqueous solutions strongly alkaline; slightly soluble in alcohol; insoluble in ether.The pentahydrate consists of flat, shiny prismatic crystals; density 1.58 g/cm3; loses three water molecules at 100°C; melts at 120°C losing all water molecules; soluble in water and alcohol; aqueous solutions strongly alkaline; insoluble in ether.The nonahydrate is a yellowish-white crystalline solid; tetragonal crystals; odor of hydrogen sulfide; the color changes on exposure to light and air, first turning to yellow and then becoming brownish-black, deliquescent; density 1.43 g/cm3; decomposes at about 50°C; very soluble in water; aqueous solution strongly alkaline; slightly soluble in alcohol; insoluble in ether. |
|
Definition |
ChEBI: A sulfide salt with formula Na2S. The pentahydrate and (particularly) the nonahydrate are also known. In gel form, sodium sulfide is used to soften toenails to assist in trimming (and so relive pain) of ingrowing toenails. |
|
General Description |
Sodium sulfide is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. If exposed to moist air Sodium sulfide is liable to spontaneous heating and may cause ignition of nearby combustible material. Sodium sulfide absorbs moisture from the air. |
|
Industrial uses |
In non-metallic flotation, sodium sulfide is also used as a depressant and for collector desorption, in particular, fatty acids from monazite, pyrochlore, zircon and microcline. As a depressant for quartz, sodium sulfide is an excellent depressant for iron-activated quartz as well as non-activated quartz. |
InChI:InChI=1/2Na.S/rNa2S/c1-3-2
1313-82-2 Relevant articles
Complex Hydroxides of Chromium: Na9[Cr(OH)6]2(OH)3 ? 6 H2O and Na4[Cr(OH)6]X ? H2O (X = Cl, (S2)1/2) - Synthesis, Crystal Structure, and Thermal Behaviour
Hinz, Dirk
, p. 1004 - 1011 (2000)
Green plate-like crystals of Na9[Cr(OH)6...
Reactions of sulphides in molten cryolite
Fellner,Korenko,Ambrová,Danielik,Thonstad
, p. 87 - 91 (2004)
The freezing point depression of cryolit...
New strategy for designing promising mid-infrared nonlinear optical materials: narrowing the band gap for large nonlinear optical efficiencies and reducing the thermal effect for a high laser-induced damage threshold
Li, Shu-Fang,Jiang, Xiao-Ming,Fan, Yu-Hang,Liu, Bin-Wen,Zeng, Hui-Yi,Guo, Guo-Cong
, p. 5700 - 5708 (2018)
To circumvent the incompatibility betwee...
Na3+xMxP1?xS4 (M = Ge4+, Ti4+, Sn4+) enables high rate all-solid-state Na-ion batteries Na2+2δFe2?δ(SO4)3|Na3+xMxP1?xS4|Na2Ti3O7
Rao, Rayavarapu Prasada,Chen, Haomin,Wong, Lee Loong,Adams, Stefan
, p. 3377 - 3388 (2017)
Electrolytes in current Na-ion batteries...
Mesolamellar thiogermanates [CnH2n + 1NH 3]4Ge4S10
Rangan, Krishnaswamy K.,Kanatzidis, Mercouri G.
, p. 4036 - 4044 (2004)
The mesostructured lamellar phases with ...
Aromatic hydrocarbon-catalyzed direct reaction of sulfur and sodium in a heterogeneous system: Selective and facile synthesis of sodium monosulfide and disulfide
Takata, Toshikazu,Saeki, Daisaku,Makita, Yoshimasa,Yamada, Nobuo,Kihara, Nobuhiro
, p. 3712 - 3714 (2003)
Sodium disulfide and monosulfide were se...
An 57Fe Moessbauer Study of the Intermediates Formed in the Reduction of FeS2 in the Li/FeS2 Battery System
Jones, C. H. W.,Kovacs, P. E.,Sharma, R. D.,McMillan, R. S.
, p. 774 - 779 (1991)
57Fe Moessbauer spectroscopy has been us...
Preparation of alkali-metal hypothiophosphates in an alcoholic solution
Svirskaya,Lupeiko,Pakhomov,Medvedeva
, p. 762 - 765 (2011)
A procedure was developed for preparatio...
Determination of structural, thermodynamic and phase properties in the Na2S-H2O system for application in a chemical heat pump
De Boer,Haije,Veldhuis
, p. 3 - 19 (2002)
Structural, thermodynamic and phase prop...
DETERMINATION OF GIBBS ENERGY OF THE EXCHANGE REACTION OF SULPHIDES USING BETA-ALUMINA SOLID ELECTROLYTE.
Itoh, Mitsuru,Kozuka, Tetsuya Yamamoto Zensaku
, (1988)
EMF measurements of the cell Na(l)/ beta...
Solvent-thermal preparation of nanocrystalline tin chalcogenide
Qian,Zhang,Wang,Wang,Xie,Qian
, p. 415 - 417 (1999)
Nanocrystalline β-SnS2 has been successf...
Zinc Tin Chalcogenide Complexes and Their Evaluation as Molecular Precursors for Cu2ZnSnS4 (CZTS) and Cu2ZnSnSe4 (CZTSe)
Fuhrmann, Daniel,Dietrich, Stefan,Krautscheid, Harald
, p. 13123 - 13131 (2017)
A series of five heteronuclear zinc tin ...
Topochemical anion metathesis routes to the Zr2N2S phases and the Na2S and ACL derivatives (A = Na, K, Rb)
Stoltz,Ramesha,Sirchio,Goenen,Eichhorn,Salamanca-Riba,Gopalakrishnan
, p. 4285 - 4292 (2003)
Anion metathesis reactions between ZrNCl...
Selective and Facile Synthesis of Sodium Sulfide and Sodium Disulfide Polymorphs
El-Shinawi, Hany,Cussen, Edmund J.,Corr, Serena A.
, p. 7499 - 7502 (2018)
Na2S and Na2S2 were selectively synthesi...
Lithium intercalation in open-ended TiS2 nanotubes
Chen, Jun,Tao, Zhan-Liang,Li, Suo-Long
, p. 2147 - 2151 (2003)
Filling the gaps: A low-temperature solu...
Flux Synthesis of LiAuS and NaAuS: Chicken-Wire-Like Layer Formation by Interweaving of (AuS)nn- Threads. Comparison with α-HgS and AAuS (A = K, Rb)
Axtell III, Enos A.,Liao, Ju-Hsiou,Kanatzidis, Mercouri G.
, p. 5583 - 5587 (1998)
From the reaction of Au with alkali meta...
Facile synthesis of thiophene/selenophene-fused acene and their optoelectronic properties
Wei, Zitang,Li, Xiangchun,Wudl, Fred,Zheng, Yonghao
, p. 7100 - 7104 (2017)
Dibenzo thiophene- and selenophene are w...
Kinetics and Mechanism of the Reaction of the Ammoniated Electron with Sodium Thiosulfate in Liquid Ammonia
Lau, Nathanielo,Dewald, Robert R.
, p. 2348 - 2350 (1980)
The reaction of sodium with thiosulfate ...
Synthesis in molten alkali metal polythiophosphate fluxes. The new quaternary bismuth and antimony thiophosphates ABiP2S7 (A = K, Rb), A3M(PS4)2 (A = K, Rb, Cs; M = Sb, Bi), Cs3Bi2(PS4)3, and Na0.16Bi1.28P2S6
McCarthy,Kanatzidis
, p. 70 - 85 (1996)
The molten alkali metal polychalcogenide...
Multielectron, Cation and Anion Redox in Lithium-Rich Iron Sulfide Cathodes
Hansen, Charles J.,Zak, Joshua J.,Martinolich, Andrew J.,Ko, Jesse S.,Bashian, Nicholas H.,Kaboudvand, Farnaz,Van Der Ven, Anton,Melot, Brent C.,Nelson Weker, Johanna,See, Kimberly A.
, p. 6737 - 6749 (2020)
Conventional Li-ion cathodes store charg...
Na2EuAs2S5, NaEuAsS4, and Na4Eu(AsS4)2: Controlling the valency of arsenic in polysulfide fluxes
Bera, Tarun K.,Kanatzidis, Mercouri G.
, p. 4293 - 4299 (2012)
The reactivity of europium with As speci...
Na3SbS3: Single crystal X-ray diffraction, raman spectroscopy, and impedance measurements
Pompe, Constantin,Pfitzner, Arno
, p. 296 - 300 (2013)
Na3SbS3 was prepared by the reaction of ...
Thiolate Spin Population of Type I Copper in Azurin Derived from33S Hyperfine Coupling
Ramirez Cohen, Marie,Mendelman, Netanel,Radoul, Marina,Wilson, Tiffany D.,Savelieff, Masha G.,Zimmermann, Herbert,Kaminker, Ilia,Feintuch, Akiva,Lu, Yi,Goldfarb, Daniella
, p. 6163 - 6174 (2017)
The electron transfer mediating properti...
Quaternary Pavonites A1+xSn2-xBi5+xS10 (A+ = Li+, Na+): Site Occupancy Disorder Defines Electronic Structure
Khoury, Jason F.,Hao, Shiqiang,Stoumpos, Constantinos C.,Yao, Zhenpeng,Malliakas, Christos D.,Aydemir, Umut,Slade, Tyler J.,Snyder, G. Jeffrey,Wolverton, Chris,Kanatzidis, Mercouri G.
, p. 2260 - 2268 (2018)
The field of mineralogy represents an ar...
Mixed thio/oxo orthovanadates Na3[VSxO4-x] (x = 2, 3): Synthesis - crystal structures - properties
Schnabel, Simone,R?hr, Caroline
, p. 479 - 490 (2005)
Mixed sodium thio/oxo orthovanadates(V),...
Neutral Mo6Q8-clusters with terminal phosphane ligands-a route to water-soluble molecular units of Chevrel phases
Gassan, Alena D.,Ivanov, Anton A.,Novikova, Evgeniya D.,Shestopalov, Michael A.,Vorotnikov, Yuri A.
, p. 2218 - 2223 (2022/02/16)
Despite the numerous studies on Chevrel ...
An efficient method for synthesizing dimethylsulfonio-34S-propionate hydrochloride from 34S8
Wirth, Joseph S.,Whitman, William B.
, p. 52 - 58 (2019/01/04)
Dimethylsulfoniopropionate (DMSP, (2-car...
1313-82-2 Process route
-

-
iron sulfide

-
-
7757-82-6
sodium sulfate

-

-
1345-25-1
iron(II) oxide

-
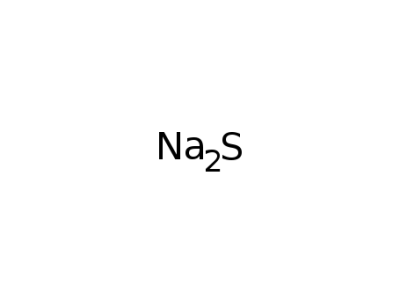
-
1313-82-2
sodium sulfide
| Conditions | Yield |
|---|---|
|
byproducts: SO2; at 590-970°C;
|
|
|
byproducts: SO2; at 590-970°C;
|
-

-
7647-14-5
sodium chloride

-
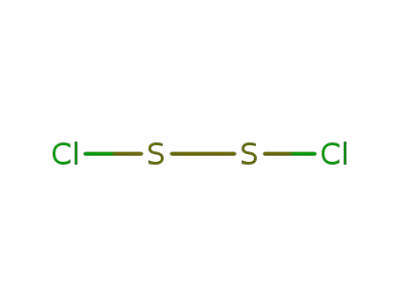
-
10025-67-9
disulfur dichloride

-
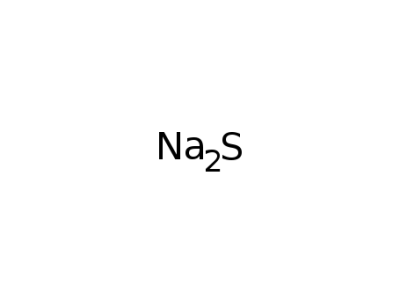
-
1313-82-2
sodium sulfide
| Conditions | Yield |
|---|---|
|
With
S;
In
melt;
reaction during melting;;
|
|
|
With
sulfur;
In
melt;
reaction during melting;;
|
1313-82-2 Upstream products
-
7440-23-5

sodium
-
7704-34-9
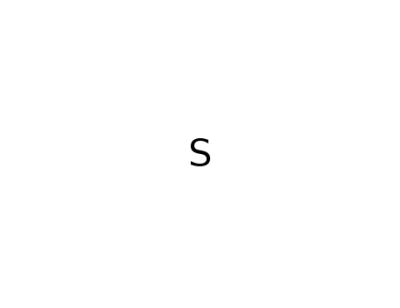
sulfur
-
7783-06-4
hydrogen sulfide
-
534-18-9
sodium trithiocarbonate
1313-82-2 Downstream products
-
22868-13-9
sodium disulfide
-
37488-76-9
sodium trisulfide
-
258872-65-0
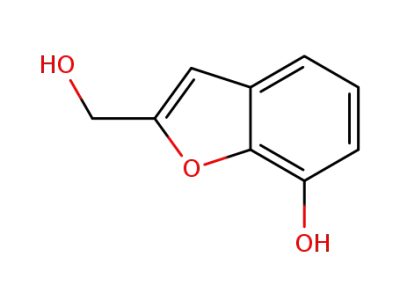
7-Hydroxy-2-hydroxymethyl-benzofuran
-
357332-70-8
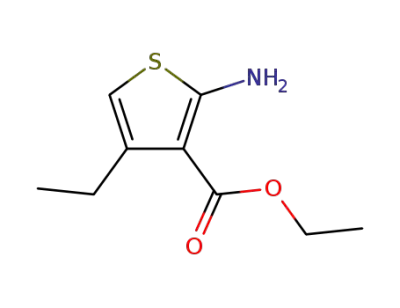
2-amino-4-methylthiophene-3-carboxylic acid ethyl ester
Relevant Products
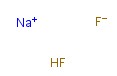
-
Sodium hydrogen fluoride
CAS:1333-83-1
-
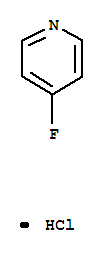
4-fluoropyridine hydrochloride
CAS:39160-31-1
-
Potassium hydrogen fluoride
CAS:7789-29-9