1597-32-6
- Product Name:2-Amino-6-fluoropyridine
- Molecular Formula:C5H5FN2
- Purity:99%
- Molecular Weight:112.107
Product Details
Manufacturers supply cost-effective and customizable 2-Amino-6-fluoropyridine 1597-32-6
- Molecular Formula:C5H5FN2
- Molecular Weight:112.107
- Vapor Pressure:0.0165mmHg at 25°C
- Melting Point:59-61 °C
- Refractive Index:1.452
- Boiling Point:224.977 °C at 760 mmHg
- PKA:2.26±0.24(Predicted)
- Flash Point:89.863 °C
- PSA:38.91000
- Density:1.257 g/cm3
- LogP:1.38410
2-Amino-6-fluoropyridine(Cas 1597-32-6) Usage
|
General Description |
2-Amino-6-fluoropyridine is a chemical compound highlighted by its distinctive structural composition where a fluorine atom and an amino group are attached to a pyridine ring in meta position. Possessing the molecular formula C5H5FN2, this compound is often used in the synthesis of various pharmaceutical and agrochemical products. Additional utility extends to the field of materials science for creation of organic semiconductors and optoelectronic devices. It's important to handle this compound with caution due to its potential for skin and eye irritation, alongside the possibility of respiratory tract irritation from inhalation. |
InChI:InChI=1/C10H9F3O2/c1-2-15-8-5-3-7(4-6-8)9(14)10(11,12)13/h3-6H,2H2,1H3
1597-32-6 Relevant articles
Peptide deformylase inhibitors
-
Page/Page column, (2014/12/09)
The present invention relates to a compo...
PEPTIDE DEFORMYLASE INHIBITORS
-
Page/Page column, (2014/02/15)
The present invention relates to a compo...
Synthesis of a novel tricyclic 1,2,3,4,4a,5,6,10b-octahydro-1,10-phenanthroline ring system and CXCR4 antagonists with potent activity against HIV-1
Catalano, John G.,Gudmundsson, Kristjan S.,Svolto, Angilique,Boggs, Sharon D.,Miller, John F.,Spaltenstein, Andrew,Thomson, Michael,Wheelan, Pat,Minick, Doug J.,Phelps, Dean P.,Jenkinson, Stephen
experimental part, p. 2186 - 2190 (2010/07/05)
Stereorandom and diastereoselective synt...
Kilogram-scale synthesis of the CXCR4 antagonist GSK812397
Boggs, Sharon,Elitzin, Vassil I.,Gudmundsson, Kristjan,Martin, Michael T.,Sharp, Matthew J.
scheme or table, p. 781 - 785 (2010/04/22)
An improved, scalable synthesis of the C...
1597-32-6 Process route
-
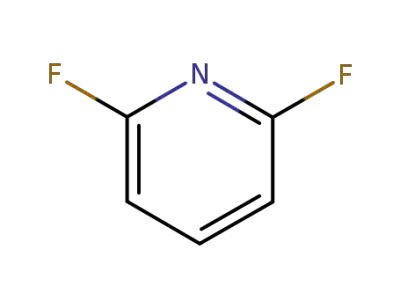
-
1513-65-1
2,6-difluoro pyridine

-
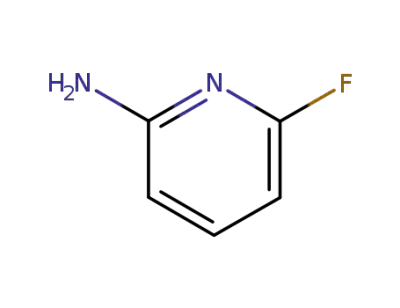
-
1597-32-6
6-fluoro-pyridin-2-ylamine
| Conditions | Yield |
|---|---|
|
With
ammonia;
In
water;
at 105 ℃;
for 15h;
|
94% |
|
With
ammonia;
In
water;
at 105 ℃;
for 15h;
|
94% |
|
With
ammonia;
In
water;
at 105 ℃;
for 15h;
|
94% |
|
With
ammonia;
In
water;
at 105 ℃;
for 15h;
|
94% |
|
With
ammonia;
In
water;
at 105 ℃;
for 15h;
|
94% |
|
With
ammonium hydroxide;
at 125 ℃;
for 5h;
under 10500.8 Torr;
|
92% |
|
With
ammonium hydroxide;
In
water;
at 110 ℃;
|
92% |
|
With
ammonium hydroxide;
at 0 - 110 ℃;
|
92% |
|
With
ammonium hydroxide;
at 125 ℃;
under 6900.69 Torr;
|
90% |
|
|
82.6% |
|
With
ammonium hydroxide;
at 105 ℃;
|
-
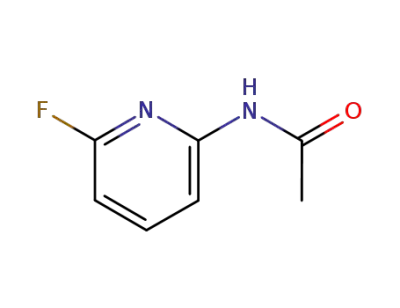
-
258343-71-4
N-(6-fluoropyridin-2-yl)acetamide

-

-
1597-32-6
6-fluoro-pyridin-2-ylamine
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
at 100 ℃;
for 4h;
|
70% |
1597-32-6 Upstream products
-
258343-71-4

N-(6-fluoropyridin-2-yl)acetamide
-
1513-65-1

2,6-difluoro pyridine
-
1075-62-3
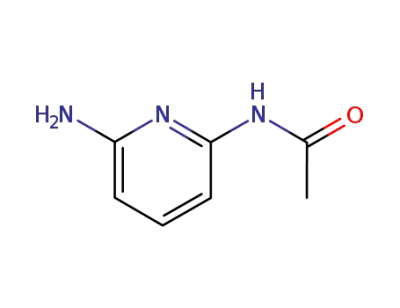
N-(6-aminopyridin-2-yl)acetamide
1597-32-6 Downstream products
-
198896-14-9
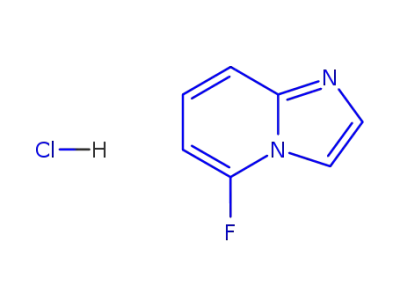
6-fluoroimidazo[1,2-a]pyridine hydrochloride salt
-
1001070-25-2
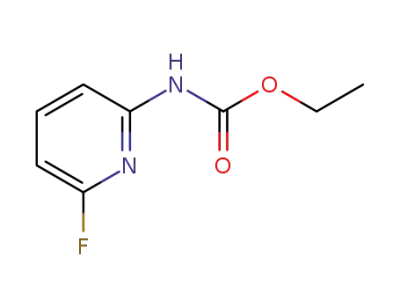
ethyl (6-fluoropyridin-2-yl)carbamate
-
878197-91-2
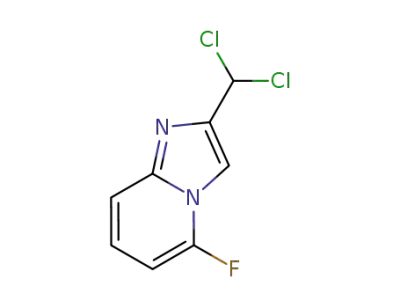
2-(dichloromethyl)-5-fluoroimidazo[1,2-a]pyridine
-
198896-12-7
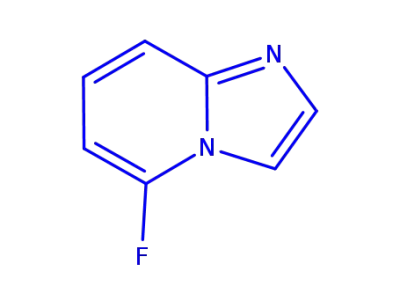
5-fluoroimidazo[1,2-a]pyridine
Relevant Products
-
5-Chloro-2-fluoropyridine
CAS:1480-65-5
-
3,4-Diaminotoluene
CAS:496-72-0
-
2,3-Diaminotoluene
CAS:2687-25-4