496-72-0
- Product Name:3,4-Diaminotoluene
- Molecular Formula:C7H10N2
- Purity:99%
- Molecular Weight:122.17
Product Details
Factory supply 3,4-Diaminotoluene 496-72-0 with sufficient stock and high standard
- Molecular Formula:C7H10N2
- Molecular Weight:122.17
- Appearance/Colour:off-white small blocks
- Vapor Pressure:0.0179mmHg at 25°C
- Melting Point:87-89 °C(lit.)
- Refractive Index:1.581
- Boiling Point:262.3 °C at 760 mmHg
- PKA:4.59±0.10(Predicted)
- Flash Point:132.1 °C
- PSA:52.04000
- Density:1.107 g/cm3
- LogP:2.32180
3,4-Diaminotoluene(Cas 496-72-0) Usage
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
3,4-Diaminotoluene neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Fire Hazard |
Flash point data for 3,4-Diaminotoluene are not available. 3,4-Diaminotoluene is probably combustible. |
|
General Description |
A colorless to brownish purple crystalline solid. Toxic by ingestion and inhalation and an irritant to skin and eyes. Soluble in water, alcohol and ether. Decomposes to emit toxic oxides of nitrogen when heated to high temperature. Used in making dyes. |
InChI:InChI=1/C8H12N2/c9-5-7-3-1-2-4-8(7)6-10/h1-4H,5-6,9-10H2
496-72-0 Relevant articles
Differential antiproliferative activity of new benzimidazole-4,7-diones
Garuti, Laura,Roberti, Marinella,Pizzirani, Daniela,Pession, Annalisa,Leoncini, Emanuela,Cenci, Valentina,Hrelia, Silvana
, p. 663 - 668 (2004)
Ten benzimidazole-4,7-diones were synthe...
Photocatalytic Oxidative [2+2] Cycloelimination Reactions with Flavinium Salts: Mechanistic Study and Influence of the Catalyst Structure
Hartman, Tomá?,Reisnerová, Martina,Chudoba, Josef,Svobodová, Eva,Archipowa, Nataliya,Kutta, Roger Jan,Cibulka, Radek
, p. 373 - 386 (2021/02/01)
Flavinium salts are frequently used in o...
Pd-Pt/modified GO as an efficient and selective heterogeneous catalyst for the reduction of nitroaromatic compounds to amino aromatic compounds by the hydrogen source
Salahshournia, Hossein,Ghiaci, Mehran
, (2019/02/14)
In this work, different nitroaromatic co...
Green synthesis and: In situ immobilization of gold nanoparticles and their application for the reduction of p -nitrophenol in continuous-flow mode
Szcs, Rózsa,Balogh-Weiser, Diána,Sánta-Bell, Evelin,Tóth-Szeles, Eszter,Varga, Tamás,Kónya, Zoltán,Poppe, László,Lagzi, István
, p. 9193 - 9197 (2019/03/28)
A green and facile method has been devel...
Novel cathepsin K inhibitors block osteoclasts in vitro and increase spinal bone density in zebrafish
Xue, Si-Tu,Wang, Ya-Li,Han, Xiao-Wan,Yi, Hong,Jiang, Wei,Si, Shu-Yi,Guo, Hui-Fang,Li, Zhuo-Rong
, p. 8600 - 8607 (2019/03/21)
Cathepsin K (Cat K) is a predominant cys...
496-72-0 Process route
-
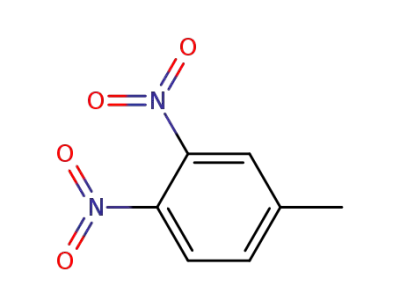
-
610-39-9
3,4-Dinitrotoluene

-
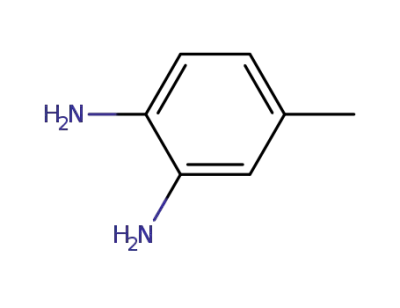
-
496-72-0
4-methyl-1,2-diaminobenzene
| Conditions | Yield |
|---|---|
|
With
5%-palladium/activated carbon; hydrogen;
In
ethanol;
at 20 ℃;
for 48h;
|
96.6% |
|
With
hydrogen;
In
methanol;
at 50 ℃;
for 0.25h;
under 1500.15 Torr;
Inert atmosphere;
|
88% |
|
With
5 wt% ruthenium/carbon; hydrogen; sodium nitrite;
In
isopropyl alcohol;
at 150 ℃;
for 4h;
under 62256.2 Torr;
Temperature;
Autoclave;
|
-
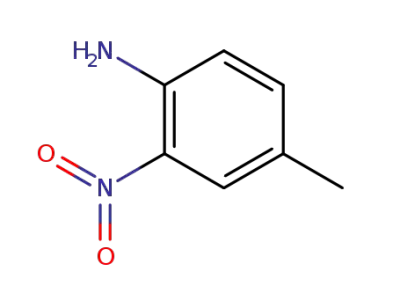
-
89-62-3
4-methyl-2-nitroaniline

-

-
496-72-0
4-methyl-1,2-diaminobenzene
| Conditions | Yield |
|---|---|
|
With
hydrogen; nickel;
In
methanol;
at 65 - 75 ℃;
for 1h;
Autoclave;
Inert atmosphere;
|
97% |
|
With
aluminum oxide; hydrazine hydrate;
iron(III) chloride;
at 111 ℃;
for 0.1h;
Irradiation;
microwave;
|
96% |
|
With
hydrazine hydrate;
In
ethanol;
at 70 ℃;
for 4h;
chemoselective reaction;
|
94% |
|
With
hydrogen;
In
methanol;
at 50 ℃;
for 0.25h;
under 1500.15 Torr;
Inert atmosphere;
|
88% |
|
With
hydrogen;
Urushibara catalyst;
In
ethanol;
at 40 - 45 ℃;
for 1.5h;
|
85% |
|
With
hydrogenchloride; tin;
for 1h;
Reflux;
|
83.5% |
|
With
ammonium formate;
10% palladium on active carbon;
In
methanol;
for 0.0833333h;
Ambient temperature;
|
79% |
|
With
sodium dithionite;
In
ethanol; water;
for 0.5h;
Reflux;
|
60% |
|
With
hydrogenchloride; tin;
|
|
|
With
sodium amalgam; potassium carbonate;
|
|
|
With
potassium carbonate; zinc;
|
|
|
With
alkali;
bei der elektrolytischen Reduktion;
|
|
|
With
platinum;
Hydrogenation;
|
|
|
With
nickel; benzene;
Hydrogenation;
|
|
|
With
hydrogen; nickel;
In
ethanol;
|
|
|
With
tin(ll) chloride;
In
methanol;
for 3h;
Heating;
|
|
|
With
10% Pd/C; hydrogen; orthoformic acid triethyl ester;
In
methanol;
at 20 ℃;
for 24h;
under 760.051 Torr;
|
|
|
With
5%-palladium/activated carbon; ammonium formate;
In
toluene;
at 120 ℃;
for 1h;
|
|
|
With
hydrazine hydrate;
In
ethanol; 1,2-dichloro-ethane;
at 30 - 50 ℃;
for 1h;
|
|
|
With
sodium tetrahydroborate;
In
water;
Kinetics;
|
|
|
With
sodium tetrahydroborate;
In
water;
for 0.05h;
|
|
|
With
palladium on activated charcoal;
In
methanol; ethyl acetate;
under 5171.62 Torr;
Flow reactor;
|
|
|
With
ammonium formate;
In
water;
at 90 ℃;
for 20h;
Inert atmosphere;
Green chemistry;
|
|
|
With
hydrazine hydrate;
In
neat (no solvent);
at 100 ℃;
for 4h;
Sealed tube;
Green chemistry;
|
|
|
With
hydrazine hydrate;
In
methanol;
Reflux;
|
|
|
With
sodium tetrahydroborate;
In
methanol;
at 30 ℃;
for 0.583333h;
|
|
|
With
sodium tetrahydroborate;
In
Triethylene glycol dimethyl ether; water;
at 20 ℃;
for 24h;
Green chemistry;
|
|
|
With
palladium on activated charcoal; hydrogen;
|
496-72-0 Upstream products
-
53476-34-9
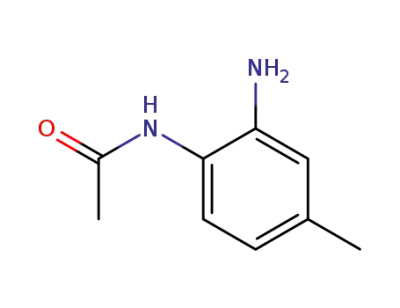
2-amino-4-methylacetanilide
-
578-46-1
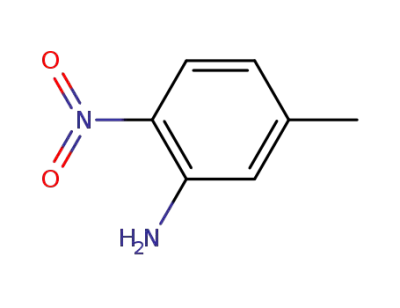
5-methyl-2-nitroaniline
-
63189-97-9
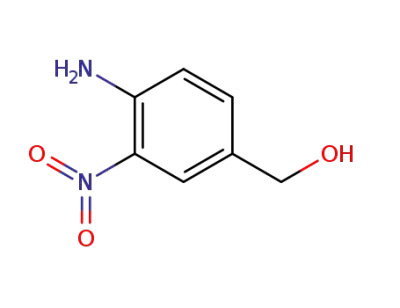
(4-amino-3-nitrophenyl)methanol
-
58010-91-6
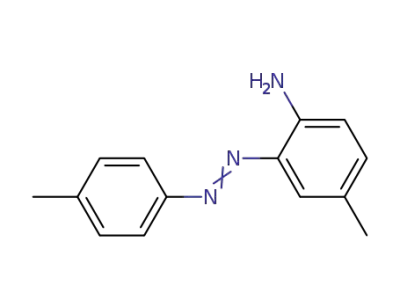
4-methyl-2-p-tolylazo-aniline
496-72-0 Downstream products
-
33138-04-4
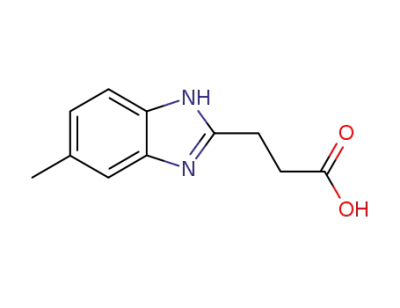
3-(5-methyl-1(3)H-benzimidazol-2-yl)-propionic acid
-
19368-41-3
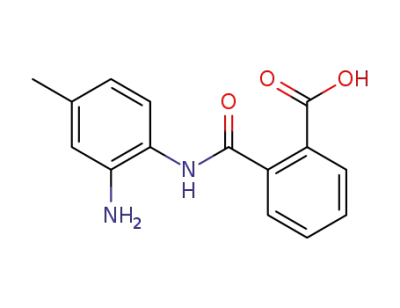
N-(2-amino-4-methyl-phenyl)-phthalamic acid
-
106242-28-8
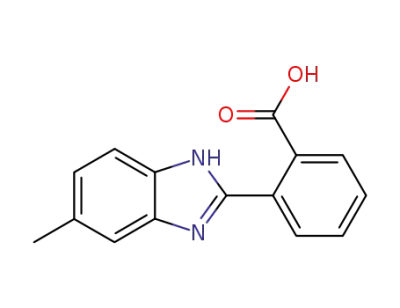
2-(5-methyl-1(3)H-benzimidazol-2-yl)-benzoic acid
-
19591-15-2
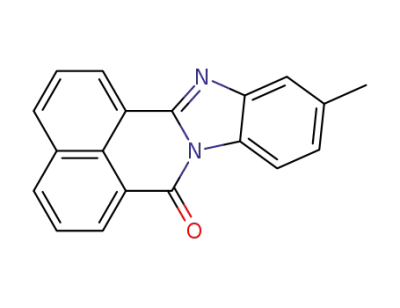
11-methyl-7H-benzimidazo[2,1-a]benz[de]isoquinolin-7-one
Relevant Products
-
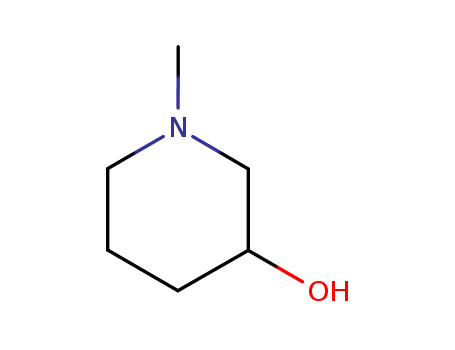
3-hydroxy-1-methylpiperidine
CAS:3554-74-3
-
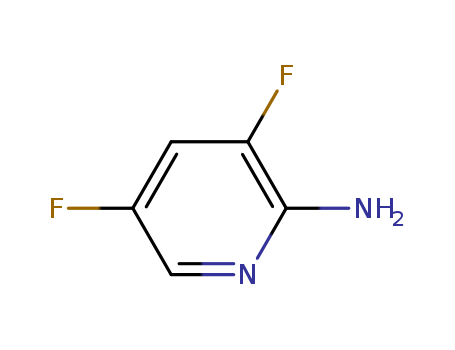
2-Amino-3,5-difluoropyridine
CAS:732306-31-9
-
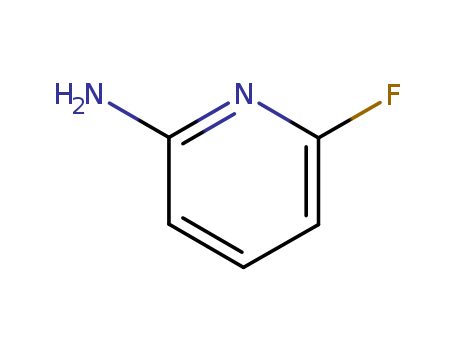
2-Amino-6-fluoropyridine
CAS:1597-32-6