105942-08-3
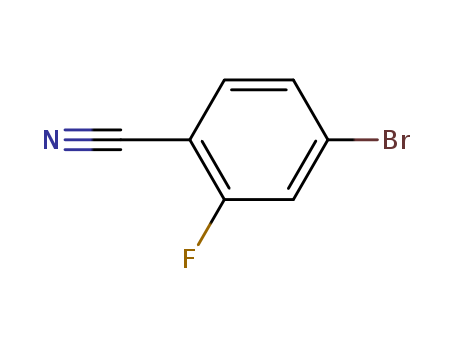
- Product Name:4-bromo-2-fluorobenzonitrile
- Molecular Formula:C7H3BrFN
- Purity:99%
- Molecular Weight:200.01
Product Details
Buy high quality and low price 4-bromo-2-fluorobenzonitrile 105942-08-3 now
- Molecular Formula:C7H3BrFN
- Molecular Weight:200.01
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.00481mmHg at 25°C
- Melting Point:69-72 °C
- Refractive Index:1.564
- Boiling Point:231.8 °C at 760 mmHg
- Flash Point:94 °C
- PSA:23.79000
- Density:1.69 g/cm3
- LogP:2.45988
4-Bromo-2-fluorobenzonitrile(Cas 105942-08-3) Usage
InChI:InChI=1/C8H5BrFN/c9-4-7-2-1-6(5-11)3-8(7)10/h1-3H,4H2
105942-08-3 Relevant articles
OXAZOLIDINONE DERIVATIVES AS ANTIMICROBIALS
-
Page/Page column 74, (2010/10/20)
The present invention relates to certain...
Further Studies on the Synthesis of Quinazolines from 2-Fluorobenzonitriles
Hynes, John B.,Tomazic, Alenka,Parrish, Christie A.,Fetzer, Oliver S.
, p. 1357 - 1364 (2007/10/02)
Recently, we reported that appropriately...
Displacement of Halogen of 2-Halogeno-Substituted Benzonitriles with Carbanions. Preparation of (2-Cyanoaryl)arylacetonitriles
Bech Sommer, Michael,Begtrup, Mikael,Boegesoe, Klaus Peter
, p. 4817 - 4821 (2007/10/02)
(2-Cyanoaryl)arylacetonitriles are obtai...
The Synthesis and Transition Temperatures of Some Fluoro-Substituted 4-Cyanophenyl and 4-Cyanobiphenyl-4'-yl 4-Pentyl- and 4-Butoxy-Benzoates
Gray, G. W.,Hird, M.,Lacey, D.,Toyne, K. J.
, p. 165 - 190 (2007/10/02)
A series of 4-cyanophenyl 4-X-benzoates ...
105942-08-3 Process route
-
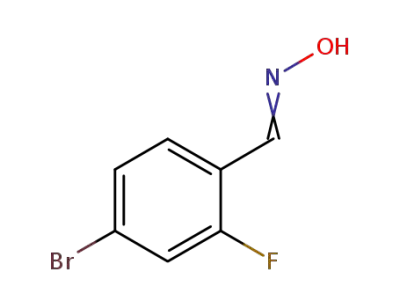
-
202865-64-3
4-bromo-2-fluorobenzaldehyde oxime

-
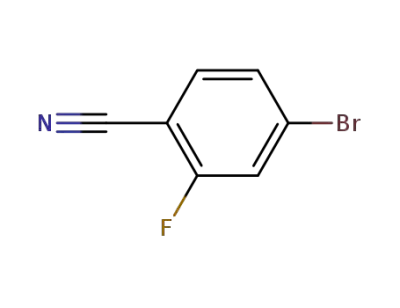
-
105942-08-3
4-bromo-6-fluorobenzonitrile
| Conditions | Yield |
|---|---|
|
With
acetic anhydride;
at 100 ℃;
for 3h;
|
-
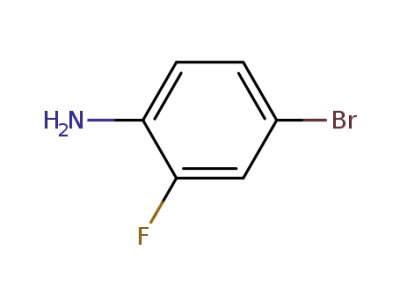
-
367-24-8
4-bromo-2-fluoroaniline

-
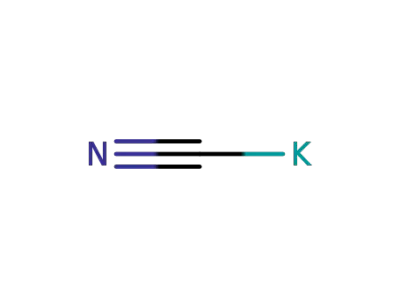
-
151-50-8
potassium cyanide

-

-
105942-08-3
4-bromo-6-fluorobenzonitrile
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; sodium hydrogencarbonate; copper(II) sulfate; sodium nitrite;
In
cyclohexane; water; acetic acid;
1.) -5 deg C, 15 min; 2.) 50 deg C;
|
76% |
|
With
hydrogenchloride; copper(l) chloride; sodium nitrite;
Yield given. Multistep reaction;
1) H2O, 0 - 5 degC, 20 min., 2) toluene, pH 6.5 (adding Na2CO3), a) 0 degC, 30 min, b) r.t, 12 h;
|
105942-08-3 Upstream products
-
367-24-8

4-bromo-2-fluoroaniline
-
544-92-3
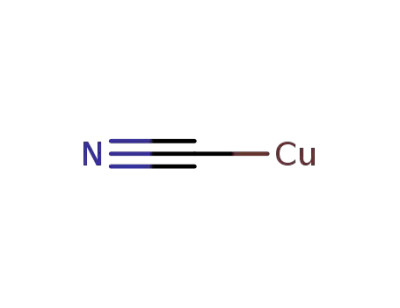
copper(I) cyanide
-
151-50-8
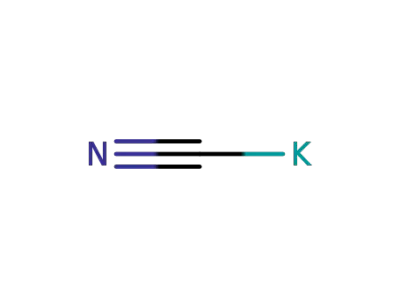
potassium cyanide
-
348-54-9
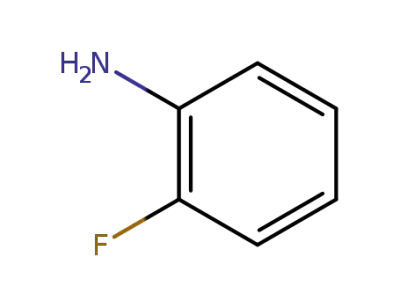
2-Fluoroaniline
105942-08-3 Downstream products
-
123864-93-7
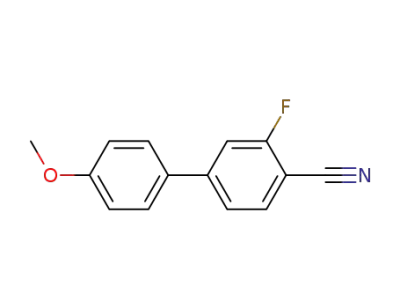
3-fluoro-4'-methoxy-1,1'-biphenyl-4-carbonitrile
-
116617-03-9
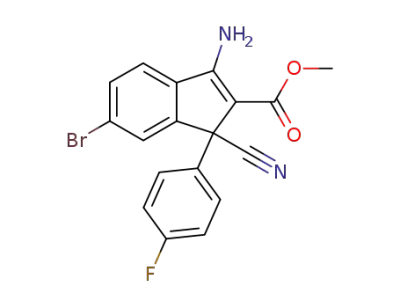
3-Amino-6-bromo-1-cyano-1-(4-fluoro-phenyl)-1H-indene-2-carboxylic acid methyl ester
-
177995-39-0
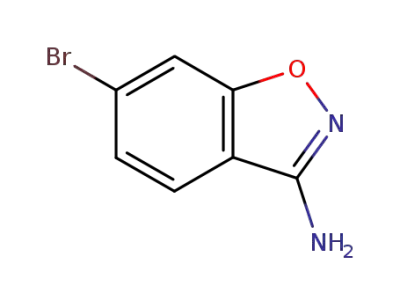
6-bromo-3-amino-1,2-benzisoxazole
-
268734-42-5
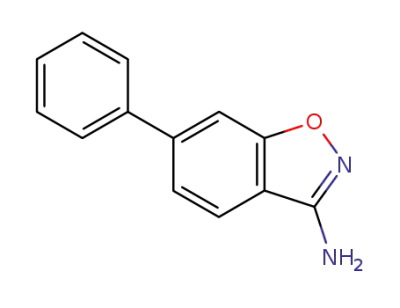
6-phenyl-3-amino-1,2-benzisoxazole
Relevant Products
-
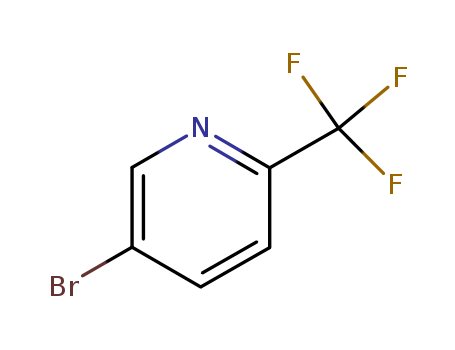
5-Bromo-2-trifluoromethylpyridine
CAS:436799-32-5
-
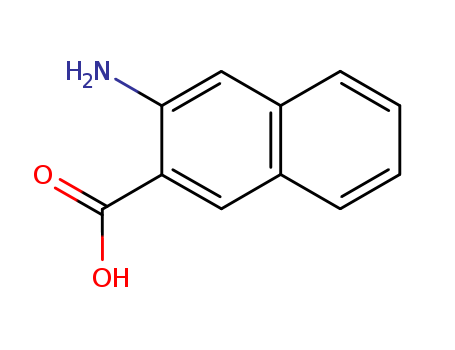
3-Amino-2-naphthoic acid
CAS:5959-52-4
-
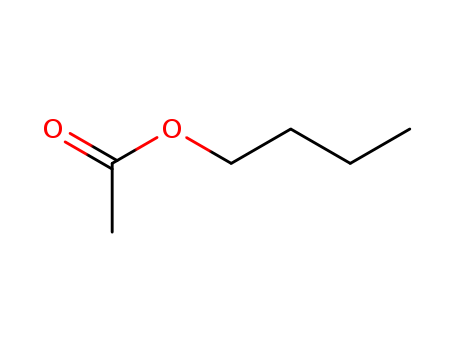
n-Butyl Acetate
CAS:123-86-4