100-37-8
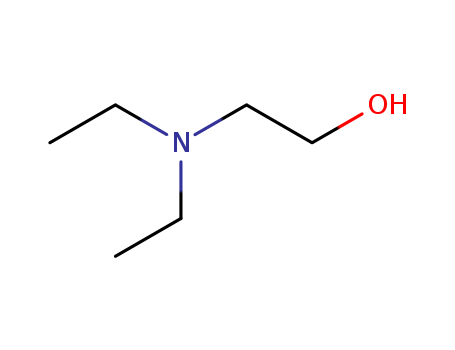
- Product Name:N,N-diethylethanolamine
- Molecular Formula:C6H15NO
- Purity:99%
- Molecular Weight:117.191
Product Details
Buy high quality and low price N,N-diethylethanolamine 100-37-8 now
- Molecular Formula:C6H15NO
- Molecular Weight:117.191
- Appearance/Colour:clear to pale yellow liquid
- Vapor Pressure:1 mm Hg ( 20 °C)
- Melting Point:-70 °C
- Refractive Index:n20/D 1.441(lit.)
- Boiling Point:164.8 °C at 760 mmHg
- PKA:14.74±0.10(Predicted)
- Flash Point:48.9 °C
- PSA:23.47000
- Density:0.884 g/cm3
- LogP:0.32050
Diethylaminoethanol(Cas 100-37-8) Usage
|
Production Methods |
2-Diethylaminoethanol (DEAE) is a tertiary amine produced by reaction of ethylene oxide or ethylene chlorhydrin and diethylamine (RTECS 1988). Itokazu (1987) has modified this process for manufacture of DEAE without eventual discoloration. Production in this country exceeds 2866 pounds per year (HSDB 1988). |
|
Air & Water Reactions |
Flammable. Soluble in water. Diethylaminoethanol is sensitive to moisture. Slowly hydrolyzes. |
|
Reactivity Profile |
Diethylaminoethanol is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Diethylaminoethanol can react with strong oxidizers and acids. |
|
Health Hazard |
INHALATION: Irritation of mucous membranes. EYES: Corrosive, causes intense pain. SKIN: Severe irritation. May cause allergic skin reaction. INGESTION: Gastrointestinal irritation.Breathing Diethylaminoethanol can irritate the nose, throat and lungs causing coughing, wheezing and/or shortness of breath. |
|
Flammability and Explosibility |
Flammable |
|
Safety |
There is a lack of data regarding human toxicity of Diethylaminoethanol. The greatest industrial hazard however, is thought to be to the eyes from contact with the fluid, which is comparable in severity to ammonium hydroxide as an eye irritant (ACGIH 1980).Diethylaminoethanol(DEAE) is permitted by the U.S. Food and Drug Administration for some applications as a food additive. Applications include protective coatings for fresh fruits and vegetables, and as an additive in steam which directly contacts food products (excluding milk products). The National Research Council Committee on Toxicology (NRC 1983) has concluded that data on long-term, low-level airborne exposures of animals to DEAE for extrapolation to human health risks are severely lacking. This, combined with the lack of data concerning the concentrations of DEAE in humidified buildings did not allow sufficient information to set guidelines for long-term exposures or estimate the health risks from such exposures. The NRC was able to make some general recommendations based on the assumption that the nitrosation reactions (below) may occur, and that the amine should be considered as hazardous as the nitroso compound formed from it. |
|
Carcinogenicity |
DEAE was not mutagenic or clastogenic in a variety of in vitro and in vivo assays. The 2003 ACGIH threshold limit valuetime- weighted average (TLV-TWA) for 2- diethylaminoethanol is 2 ppm (9.6mg/m3) with a notation for skin absorption. |
|
Environmental Fate |
DEAE, when compared with other amino alcohols, was observed to be biologically undecomposable in an experiment using activated sludge (HSDB 1988). |
|
Metabolism |
The absorption of DEAE (administered orally as DEAE acid malate or 'Cerebrol') in healthy adult rats is very rapid, reaching a peak plasma level in 30 min (Bismut et al 1986). The biological half-life is 3.5 h with 39% of the excreted product appearing in the urine after 48 h (Bismut et al 1986). In an earlier study, Schulte et al (1972) demonstrated that in rats, following a single oral dose, excretion occurs mainly through the kidneys with 37-59% being eliminated in the first 24 h. After 48 h, elimination was independent of dose. The brain and spinal cord showed the highest concentration after 7 d. Metabolites produced were observed to be diethylaminoethanol N-oxide, diethylaminoacetic acid, and ethylaminoethanol. |
|
Physical properties |
Colorless, hygroscopic liquid with a nauseating, ammonia-like odor. Experimentally determined detection and recognition odor threshold concentrations were 50 μg/m3 (11 ppbv) and 190 μg/m3 (40 ppbv), respectively (Hellman and Small, 1974). |
|
Application |
Diethylethanolamine is used as a corrosion inhibitor in steam and condensate lines by neutralizing carbonic acid and scavenging oxygen. It use as a chemical intermediate for production of emulsifiers, detergents, and solubilizers. DEAE is also an intermediate for manufacturing cosmetics; textile finishing agents, fabric softeners, and dyes; drugs and pharmaceuticals, and fatty acid. It is also used in antirust compositions, and acts as a curing agent for resins. |
|
Definition |
ChEBI: 2-diethylaminoethanol is a member of the class of ethanolamines that is aminoethanol in which the hydrogens of the amino group are replaced by ethyl groups. It is a member of ethanolamines, a tertiary amino compound and a primary alcohol. It derives from an ethanolamine. It derives from a hydride of a triethylamine. |
|
General Description |
A colorless liquid. Flash point 103-140°F. Less dense than water . Vapors heavier than air. Produces toxic oxides of nitrogen during combustion. Causes burns to the skin, eyes and mucous membranes. |
|
Industrial uses |
Diethylaminoethanol(DEAE) is used in the pharmaceutical industry for the manufacture of the local anesthetics procaine and chloroquine; and in the chemical industry for the manufacture of water-soluble salts, fatty-acid derivatives, derivatives containing tertiary amine groups, emulsifiers, special soaps, cosmetics and textiles and fibers (HSDB 1988). It also is used in chromatography in chemistry and biochemistry laboratories (DEAE is useful as an ion-exchange matrix; DEAE-cellulose columns are used for purification of proteins and DNA, and DEAE-silica for phospholipid separations). In other industries DEAE is used in some antirust compositions and in textile softeners (Hawley 1977; HSDB 1988). It is also used widely as a steam additive in large buildings requiring humidifiers. |
InChI:InChI=1/C6H15NO/c1-4-7(5-2)6(3)8/h6,8H,4-5H2,1-3H3
100-37-8 Relevant articles
-
Kornet,M.J. et al.
, p. 3637 - 3639 (1968)
-
3H2-amyxin synthesis and isolation from biological substrates
Karpinchik,Mal'tsev,Sumrii
, p. 448 - 450 (2002)
-
Hydrogenolysis of Amide Acetals and Iminium Esters
Kadyrov, Renat
, p. 170 - 172 (2018)
Amide acetals and iminium esters were hy...
-
Bronnert,D.L.E.,Saunders,B.C.
, p. 160 - 163 (1960)
-
Synthesis and Antibacterial Activity of Polymerizable Acryloyloxyalkyltriethyl Ammonium Salts
Mancuso, Raffaella,Amuso, Roberta,Armentano, Biagio,Grasso, Giuseppe,Rago, Vittoria,Cappello, Anna Rita,Galiano, Francesco,Figoli, Alberto,De Luca, Giorgio,Hoinkis, Jan,Gabriele, Bartolo
, p. 1235 - 1244 (2017)
This study reports an efficient and prac...
Homogeneously Catalyzed Synthesis of β-Amino Alcohols ans Vicinal Diamines from Ethylene Glycol and 1,2-Propanediol
Marsella, John A.
, p. 467 - 468 (1987)
Disubstituted β-amino alcohols and tetra...
Kinetics of hydrolysis of procaine in aqueous and micellar media
Al-Blewi, Fawzia Faleh,Al-Lohedan, Hamad A.,Rafiquee,Issa, Zuheir A.
, p. 1 - 9 (2013)
The kinetics of alkaline hydrolysis of p...
-
Cadogan
, p. 18,20 (1962)
-
Inclusion complexation of novocaine by β-cyclodextrin in aqueous solutions
Iglesias, Emilia
, p. 4383 - 4392 (2006)
The formation of inclusion complexes bet...
A method for preparing alkyl amine
-
Paragraph 0102-0105, (2017/07/12)
The invention discloses a preparation me...
Design, Synthesis, and Biological Evaluation of Scutellarein Derivatives Based on Scutellarin Metabolic Mechanism in Vivo
Dong, Ze-Xi,Shi, Zhi-Hao,Li, Nian-Guang,Zhang, Wei,Gu, Ting,Zhang, Peng-Xuan,Wu, Wen-Yu,Tang, Yu-Ping,Fang, Fang,Xue, Xin,Li, He-Min,Cheng, Hai-Bo,Yang, Jian-Ping,Duan, Jin-Ao
, p. 946 - 957 (2016/05/24)
Three series of scutellarein derivatives...
100-37-8 Process route
-
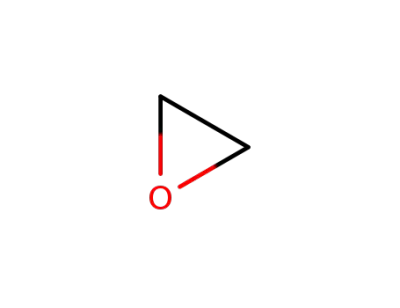
-
75-21-8,99932-75-9
oxirane

-
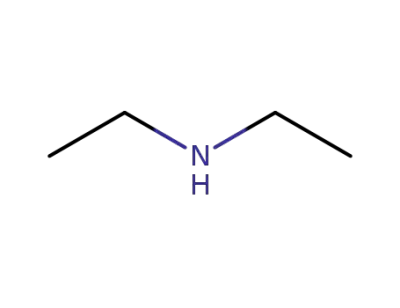
-
109-89-7
diethylamine

-
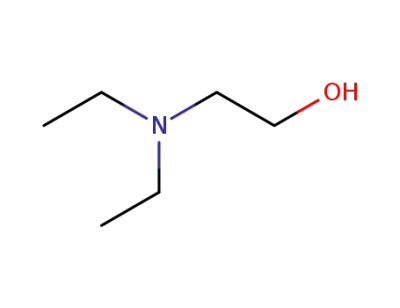
-
100-37-8
2-(Diethylamino)ethanol
| Conditions | Yield |
|---|---|
|
With
3-amino-2-propanol;
at 70 ℃;
for 3h;
under 4500.45 Torr;
Pressure;
Temperature;
|
99.4% |
|
With
water;
|
|
|
water;
at 85.84 - 94.84 ℃;
for 0.116667h;
under 1500.15 Torr;
Heating / reflux;
|
|
|
at 76.84 - 112.84 ℃;
for 0.133333h;
under 1500.15 Torr;
Heating / reflux;
|
-
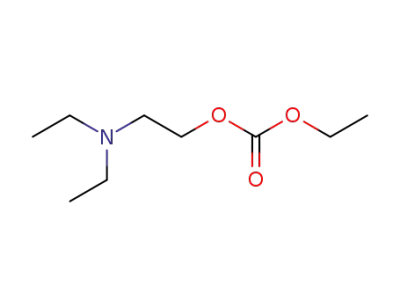
-
20570-43-8
carbonic acid ethyl ester-(2-diethylamino-ethyl ester)

-

-
100-37-8
2-(Diethylamino)ethanol

-
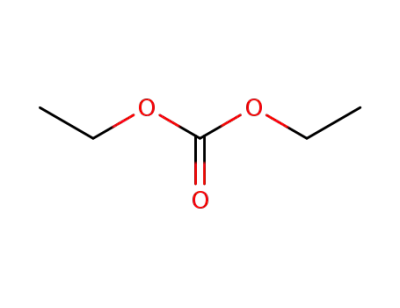
-
105-58-8,76619-83-5
Diethyl carbonate
| Conditions | Yield |
|---|---|
|
|
100-37-8 Upstream products
-
75-21-8

oxirane
-
109-89-7

diethylamine
-
20570-43-8

carbonic acid ethyl ester-(2-diethylamino-ethyl ester)
-
64-67-5
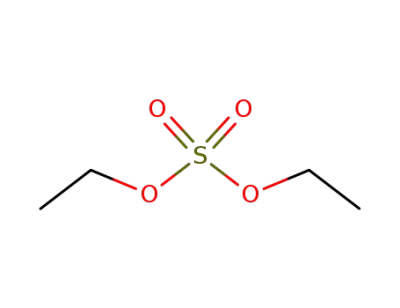
diethyl sulfate
100-37-8 Downstream products
-
140-82-9
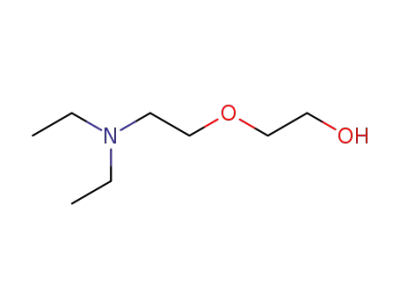
2-[2-(diethylamino)ethoxy] ethanol
-
75-07-0
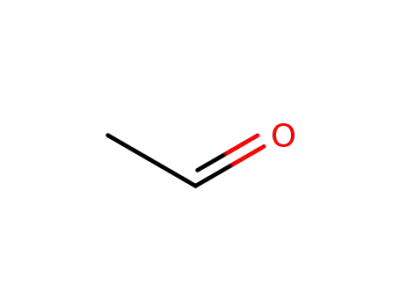
acetaldehyde
-
109-89-7

diethylamine
-
110-73-6
2-(Ethylamino)ethanol
Relevant Products
-
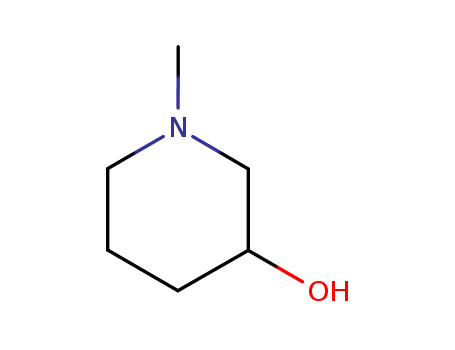
3-hydroxy-1-methylpiperidine
CAS:3554-74-3
-
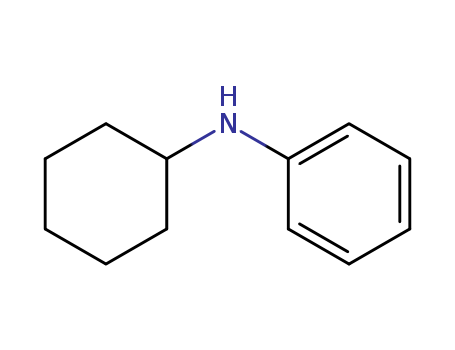
N-cyclohexylbenzenamine
CAS:1821-36-9
-
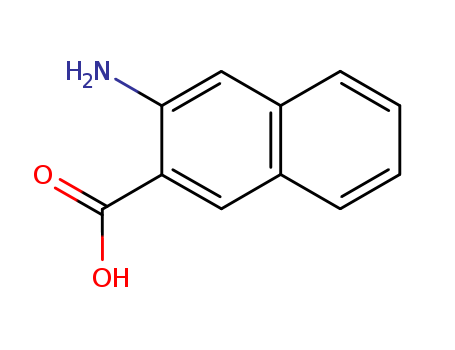
3-Amino-2-naphthoic acid
CAS:5959-52-4