1821-36-9
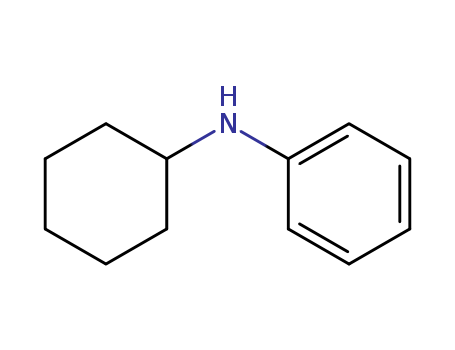
- Product Name:N-cyclohexylbenzenamine
- Molecular Formula:C12H17N
- Purity:99%
- Molecular Weight:175.274
Product Details
Buy cost-effective 99% pure N-cyclohexylbenzenamine 1821-36-9 now
- Molecular Formula:C12H17N
- Molecular Weight:175.274
- Appearance/Colour:dark brown liquid
- Vapor Pressure:0.00178mmHg at 25°C
- Melting Point:14-15 °C
- Refractive Index:n20/D 1.560
- Boiling Point:292.9 °C at 760 mmHg
- PKA:5.46±0.20(Predicted)
- Flash Point:135.5 °C
- PSA:12.03000
- Density:1.022 g/cm3
- LogP:3.50420
N-CYCLOHEXYLANILINE(Cas 1821-36-9) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 50, p. 1927, 1985 DOI: 10.1021/jo00211a028Synthetic Communications, 19, p. 565, 1989Tetrahedron Letters, 16, p. 119, 1975 |
InChI:InChI=1/C12H17N/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h1,3-4,7-8,12-13H,2,5-6,9-10H2
1821-36-9 Relevant articles
Synthesis and characterization of bidentate NHC-Pd complexes and their role in amination reactions
Demir, Serpil,?zdemir, Ismail,?etinkaya, Bekir,Arslan, Hakan,VanDerveer, Don
, p. 195 - 200 (2011)
The new well-defined and air-stable orth...
Reduction of imines to amines through use of Cp2MoH2 and protonic acid system
Minato, Makoto,Fujiwara, Yutaka,Ito, Takashi
, p. 647 - 648 (1995)
Imines are conveniently reduced to the c...
THE ACTUAL MERCURATING SPECIES IN THE MERCURATION OF AROMATIC AMINES AND THE AMINOMERCURATION OF OLEFINS
Barluenga, Jose,Bayon, Ana M.,Perez-Prieto, Julia,Asensio, Gregorio
, p. 5053 - 5062 (1984)
The reactivity of ?- and ?- N-mercurated...
Highly Coordinated Tin Hydrides: A Novel Synthesis of Tertiary Amines via Hydrostannation of Imines
Kawakami, Takayo,Sugimoto, Takayuki,Shibata, Ikuya,Baba, Akio,Matsuda, Haruo,Sonoda, Noboru
, p. 2677 - 2682 (1995)
The highly coordinated tin hydride, Bu2S...
ELECTROLYTIC BEHAVIOR OF TETRASUBSTITUTED IMINIUM SALT IN ACETONITRILE.
Kunai,Harada,Nishihara,Yanagi,Sasaki
, p. 2442 - 2446 (1983)
Cathodic behavior of N-cyclohexylidenepy...
Synthesis of palladium complexes derived from imidazolidin-2-ylidene ligands and used for catalytic amination reactions
Karaca, Emine ?zge,Gürbüz, Nevin,?ahin, Onur,Büyükgüng?r, Orhan,?zdemir, ?smail
, p. 1050 - 1055 (2016)
N-Aryl amination and the Buchwald–Hartwi...
Oxidative three-component carboamination of vinylarenes with alkylboronic acids
Gockel, Samuel N.,Lee, SangHyun,Gay, Brittany L.,Hull, Kami L.
, p. 5166 - 5171 (2021)
The three-component carboamination of al...
The impact of the nature of amine reactants in the palladium catalyzed conversion of phenol to N-substituted anilines
Tomkins, Patrick,Valgaeren, Carlot,Adriaensen, Koen,Cuypers, Thomas,De Vos, Dirk E.
, p. 207 - 213 (2019)
Anilines and cyclohexylamines are curren...
Visible-light-mediated tungsten-catalyzed C-H amination of unactivated alkanes with nitroarenes
Wang, Qing,Ni, Shengyang,Wang, Xiaochen,Wang, Yi,Pan, Yi
, p. 678 - 685 (2022/02/14)
Alkylamines are important motifs in phar...
Imine reduction with me2s-bh3
Kamal, Mohammad M.,Liu, Zhizhou,Vidovi?, Dragoslav,Zhai, Siyuan
, (2021/09/13)
Although there exists a variety of diffe...
Sulfated polyborate: A dual catalyst for the reductive amination of aldehydes and ketones by NaBH4
Ganwir, Prerna,Chaturbhuj, Ganesh
supporting information, (2021/05/19)
An efficient, quick, and environment-fri...
Palladium supported on magnesium hydroxyl fluoride: An effective acid catalyst for the hydrogenation of imines and N-heterocycles
Agbossou-Niedercorn, Francine,Corre, Yann,Dongare, Mohan K.,Kemnitz, Erhard,Kokane, Reshma,Michon, Christophe,Umbarkar, Shubhangi B.
supporting information, p. 19572 - 19583 (2021/11/04)
Palladium catalysts supported on acidic ...
1821-36-9 Process route
-
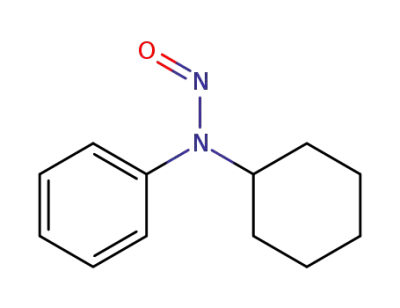
-
54955-24-7
N-cyclohexyl-N-nitrosoaniline

-
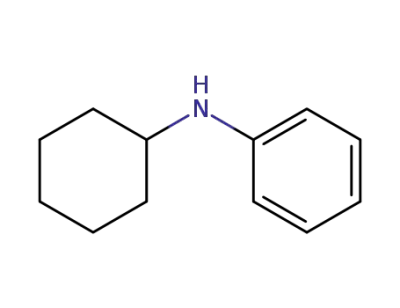
-
1821-36-9
N-phenyl-2-cyclohexylamine

-
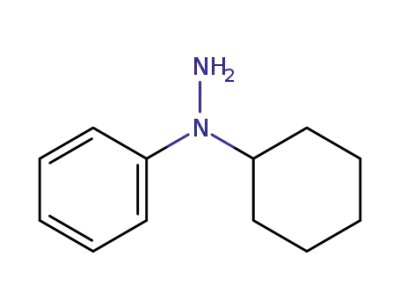
-
77612-47-6
1-cyclohexyl-1-phenylhydrazine
| Conditions | Yield |
|---|---|
|
With
acetic acid; zinc;
|
-
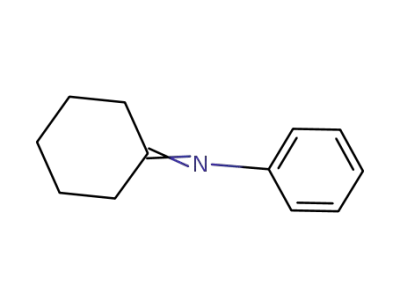
-
1132-38-3
N-cyclohexylidenebenzenamine

-

-
1821-36-9
N-phenyl-2-cyclohexylamine
| Conditions | Yield |
|---|---|
|
With
Bu2SnClH-HMPA;
In
tetrahydrofuran;
for 1h;
Ambient temperature;
|
97% |
|
With
triethylsilane; palladium diacetate;
In
ethanol;
for 0.5h;
Inert atmosphere;
|
90% |
|
With
bis(η5-cyclopentadinyl)dihydridomolybdenum; acetic acid;
In
toluene;
for 34h;
Ambient temperature;
|
89% |
|
With
acetic acid; acetophenone;
bis(η5-cyclopentadinyl)dihydridomolybdenum;
In
toluene;
Ambient temperature;
|
88% |
|
With
trichloroacetic acid;
In
toluene;
at 20 ℃;
for 2.5h;
Inert atmosphere;
|
88% |
|
With
hydrogen; lithium hexamethyldisilazane;
In
toluene;
at 80 ℃;
under 75007.5 Torr;
Inert atmosphere;
Autoclave;
|
83% |
|
With
dimethylsulfide borane complex;
In
chloroform-d1;
at 60 ℃;
for 6h;
chemoselective reaction;
Sealed tube;
Schlenk technique;
|
81% |
|
With
aluminum isopropoxide; nickel; isopropyl alcohol;
In
xylene;
for 1h;
Heating;
|
80% |
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; methanol;
at -10 ℃;
for 2h;
|
74% |
|
With
sodium tetrahydroborate; hydrogen; nickel dichloride;
In
isopropyl alcohol;
at 70 ℃;
for 8h;
under 760.051 Torr;
|
69% |
|
With
tetraethylammonium perchlorate; benzoic acid;
In
acetonitrile;
electrolysis;
|
66% |
|
With
triethylsilane; [(η5-pentamethylcyclopentadienyl)Ir(2-phenylpyridine(1-))Cl]; sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate;
In
dichloromethane;
at 25 ℃;
for 0.25h;
Inert atmosphere;
|
37% |
|
With
dicobalt octacarbonyl; carbon monoxide; benzene;
at 135 - 140 ℃;
under 132391 Torr;
Hydrogenation;
|
|
|
With
potassium hydroxide; trichlorosilane;
Yield given. Multistep reaction;
1) BF3*Et2O, benzene, 4 h, reflux, 2) 80percent aq. EtOH, 12 h, r.t.;
|
|
|
With
potassium hydroxide; trichlorosilane;
Yield given. Multistep reaction;
1.) acetonitrile, reflux, 4 h, 2.) EtOH, RT, 12 h;
|
|
|
With
sodium tetrahydroborate; Montmorillonite K10 clay; water;
for 0.0166667h;
microwave irradiation;
|
|
|
With
potassium formate;
palladium diacetate;
In
N,N-dimethyl-formamide;
at 50 ℃;
for 5h;
|
|
|
With
sodium tetrahydroborate; HZSM-5 zeolite; water;
for 0.0833333h;
microwave irradiation;
|
|
|
With
sodium tetrahydroborate; trifluoroacetic acid;
In
tetrahydrofuran; N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
13 mg |
|
With
methanol; sodium tetrahydroborate;
at 0 - 20 ℃;
for 1h;
Reagent/catalyst;
Inert atmosphere;
|
31.5 mg |
|
Multi-step reaction with 2 steps
1: cyclohexylamine; hydrogen; water / diethyl ether / 20 h / 59.84 °C / 6000.6 Torr
2: hydrogen; water; 5% Pd/C / diethyl ether / 20 h / 59.84 °C / 6000.6 Torr
With
5% Pd/C; water; hydrogen; cyclohexylamine;
In
diethyl ether;
|
|
|
Multi-step reaction with 2 steps
1: cyclohexylamine; hydrogen; water; 5% Pd/C / diethyl ether / 20 h / 59.84 °C / 6000.6 Torr
2: hydrogen; water; 5% Pd/C / diethyl ether / 20 h / 59.84 °C / 6000.6 Torr
With
5% Pd/C; water; hydrogen; cyclohexylamine;
In
diethyl ether;
|
|
|
With
hydrogen;
In
isopropyl alcohol;
at 80 ℃;
for 13h;
under 7500.75 Torr;
Autoclave;
|
> 99 %Spectr. |
|
With
sodium tetrahydroborate;
In
ethanol;
at 70 ℃;
Green chemistry;
|
|
|
With
hydrogen;
In
methanol;
at 60 ℃;
for 48h;
under 7500.75 Torr;
Catalytic behavior;
|
91 %Chromat. |
1821-36-9 Upstream products
-
54955-24-7

N-cyclohexyl-N-nitrosoaniline
-
1132-38-3

N-cyclohexylidenebenzenamine
-
108-94-1
cyclohexanone
-
98-95-3
nitrobenzene
1821-36-9 Downstream products
-
4212-82-2
N-cyclohexyl-acetanilide
-
110-83-8
cyclohexene
-
56418-80-5
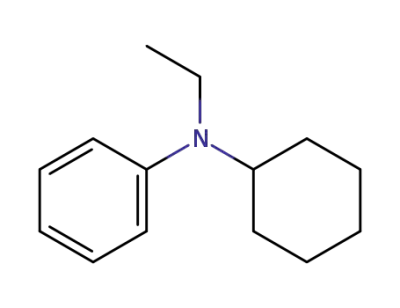
N-cyclohexyl-N-ethylaniline
-
4705-13-9
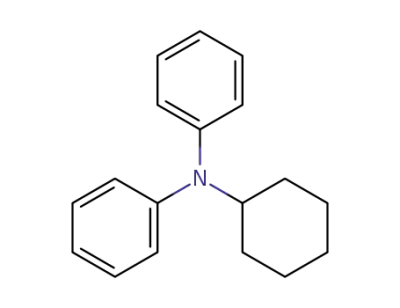
N-cyclohexyl-N-phenylaniline
Relevant Products
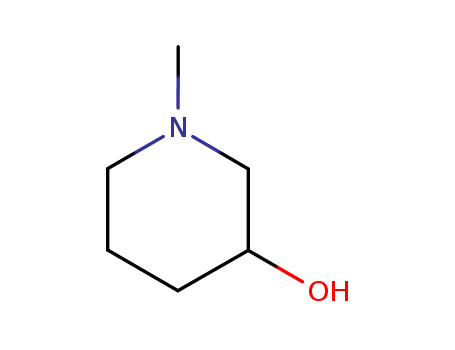
-
3-hydroxy-1-methylpiperidine
CAS:3554-74-3
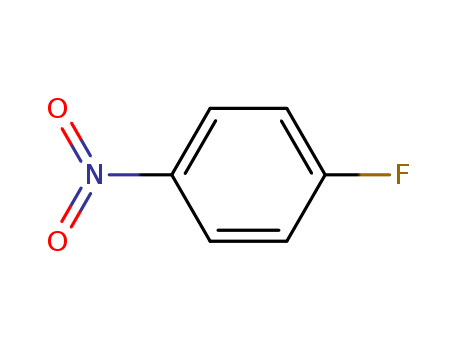
-
4-Fluoronitrobenzene
CAS:350-46-9
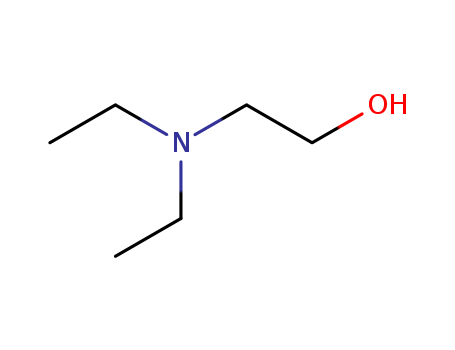
-
N,N-diethylethanolamine
CAS:100-37-8