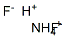
18144-47-3
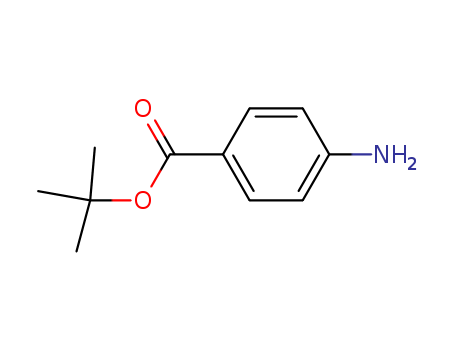
- Product Name:tert-Butyl 4-aminobenzoate
- Molecular Formula:C11H15NO2
- Purity:99%
- Molecular Weight:193.246
Product Details
Manufacturer supply top purity tert-Butyl 4-aminobenzoate 18144-47-3 with GMP standards
- Molecular Formula:C11H15NO2
- Molecular Weight:193.246
- Vapor Pressure:0.000279mmHg at 25°C
- Melting Point:108-110 °C
- Refractive Index:1.538
- Boiling Point:322.4 °C at 760 mmHg
- PKA:2.43±0.10(Predicted)
- Flash Point:173.6 °C
- PSA:52.32000
- Density:1.078 g/cm3
- LogP:2.80530
tert-Butyl 4-aminobenzoate(Cas 18144-47-3) Usage
|
Uses and Mechanism of Action |
Tert-Butyl 4-Aminobenzoate falls under the category of pharmaceutical intermediates, other intermediates, and synthetic material intermediates. Tert-Butyl 4-Aminobenzoate is used as a reagent in peptide synthesis. It serves as a research reagent in pharmaceutical development and drug discovery processes. |
|
Production Methods |
Tert-Butyl 4-Aminobenzoate can be synthesized through various chemical reactions, including amination of tert-butyl 4-bromobenzoate using palladium-catalyzed reactions. |
|
Analysis Method |
The purity and characteristics of Tert-Butyl 4-Aminobenzoate can be analyzed using techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), and infrared spectroscopy (IR). Additionally, precautions should be taken during handling to avoid personal contact and exposure to ignition sources due to its potential explosive properties. |
|
General Description |
Tert-Butyl 4-aminobenzoate, also known as butamben, is a chemical compound with the formula C11H15NO2. It's an ester and a tertiary amine, used primarily as a local anesthetic. As an active ingredient in some topical and ophthalmic medications, it can provide pain relief by blocking sodium channels and therefore preventing nerve impulses. It is characterized by its off-white appearance and slight aromatic smell. Considering safety, it is generally considered low-hazard but can cause eye and skin irritation if not properly handled. Proper storage and disposal methods should be followed. |
InChI:InChI=1/C11H15NO2/c1-11(2,3)14-10(13)8-4-6-9(12)7-5-8/h4-7H,12H2,1-3H3
18144-47-3 Relevant articles
Hydroboration reduction reaction of aromatic nitro compounds without transition metal catalysis
-
Paragraph 0006; 0069-0072, (2021/07/31)
The invention relates to a hydroboration...
Innovative Multipodal Ligands Derived from Tr?ger's Bases for the Sensitization of Lanthanide(III) Luminescence
Barja, Beatriz C.,Bruttomesso, Andrea C.,Eliseeva, Svetlana V.,Petoud, Stéphane,Ramírez, Javier A.,Trupp, Leandro,Vardé, Mariana
supporting information, p. 16900 - 16909 (2020/11/30)
Herein, the synthesis and characterizati...
Synergistic effects in Fe nanoparticles doped with ppm levels of (Pd + Ni). A new catalyst for sustainable nitro group reductions
Pang, Haobo,Gallou, Fabrice,Sohn, Hyuntae,Camacho-Bunquin, Jeffrey,Delferro, Massimiliano,Lipshutz, Bruce H.
supporting information, p. 130 - 135 (2018/01/12)
A remarkable synergistic effect has been...
Carbonyl Iron Powder: A Reagent for Nitro Group Reductions under Aqueous Micellar Catalysis Conditions
Lee, Nicholas R.,Bikovtseva, Agata A.,Cortes-Clerget, Margery,Gallou, Fabrice,Lipshutz, Bruce H.
supporting information, p. 6518 - 6521 (2017/12/26)
An especially mild, safe, efficient, and...
18144-47-3 Process route
-
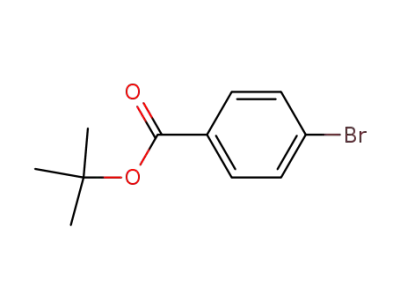
-
59247-47-1
tert-butyl-4-bromobenzoate

-
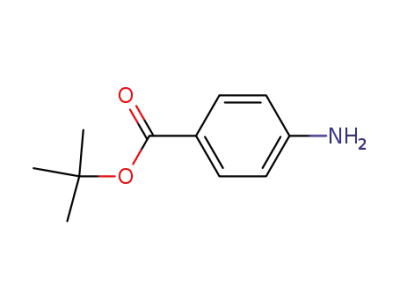
-
18144-47-3
tert-butyl 4-aminobenzoate

-
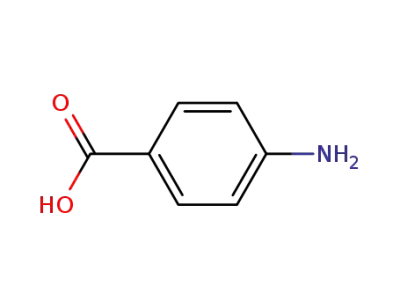
-
150-13-0,159246-81-8,8014-65-1
4-amino-benzoic acid
| Conditions | Yield |
|---|---|
|
tert-butyl-4-bromobenzoate;
With
polystyrene Rink amine resin; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate;
tris-(dibenzylideneacetone)dipalladium(0);
In
1,4-dioxane; tert-butyl alcohol;
at 80 ℃;
for 20h;
With
trifluoroacetic acid;
In
dichloromethane;
for 0.75h;
|
21% |
-
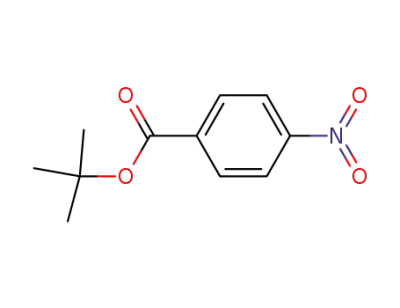
-
19756-72-0
tert-butyl 4-nitrobenzoate

-

-
18144-47-3
tert-butyl 4-aminobenzoate
| Conditions | Yield |
|---|---|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
|
100% |
|
With
hydrogen;
palladium on activated charcoal;
In
methanol;
for 5h;
|
100% |
|
With
hydrogen;
AV-17-8; palladium;
In
ethanol;
at 45 ℃;
under 750.06 Torr;
|
99% |
|
With
iron; ammonium chloride;
In
ethanol; water;
at 95 ℃;
|
99% |
|
With
triethyl borane; potassium tert-butylate; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane;
In
tetrahydrofuran;
at 100 ℃;
for 24h;
Sealed tube;
Inert atmosphere;
|
99% |
|
With
ammonium chloride;
In
tetrahydrofuran; water;
at 45 ℃;
for 5h;
Sealed tube;
Green chemistry;
|
98% |
|
With
sodium tetrahydroborate; TPGS-750-M;
In
tetrahydrofuran; water;
at 20 ℃;
for 2h;
|
98% |
|
With
hydrogen;
5%-palladium/activated carbon;
In
methanol;
at 20 ℃;
for 3h;
under 1551.49 Torr;
|
90% |
|
With
hydrogen;
palladium on activated charcoal;
In
tetrahydrofuran; water;
at 20 ℃;
for 19h;
under 2740.88 Torr;
|
80% |
|
With
water; iron;
Heating;
|
|
|
With
hydrogen;
palladium on activated charcoal;
In
ethyl acetate;
|
|
|
With
hydrogen;
AV-17-8-Pd;
In
ethanol;
at 45 ℃;
Yield given;
|
|
|
With
hydrogen;
AV-17-8; palladium;
In
ethanol;
at 45 ℃;
under 750.06 Torr;
Rate constant;
other Pd catalysts;
|
|
|
With
hydrogen;
palladium on activated charcoal;
In
ethanol;
|
|
|
With
hydrogen;
palladium on activated charcoal;
|
|
|
With
hydrogen;
1% Pd/C;
|
|
|
With
hydrogen;
palladium 10% on activated carbon;
In
tetrahydrofuran; methanol;
for 2h;
under 3102.97 Torr;
|
18144-47-3 Upstream products
-
19756-72-0

tert-butyl 4-nitrobenzoate
-
24537-25-5
4-sulfinylamino-benzoyl chloride
-
75-65-0
tert-butyl alcohol
-
59247-47-1

tert-butyl-4-bromobenzoate
18144-47-3 Downstream products
-
94930-28-6
N-<4'-(tert-butoxycarbonyl)phenyl>-3-pyrrolidinol
-
108292-73-5
Benzyl 2,3-Anhydro-4-<4-(tert-butyloxycarbonyl)phenylamino>-4-deoxy-β-L-lyxopyranoside
-
108292-74-6
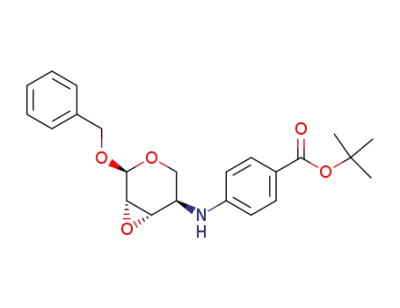
Benzyl 2,3-Anhydro-4-<4-(tert-butyloxycarbonyl)phenylamino>-4-deoxy-α-D-lyxopyranoside
-
108292-75-7
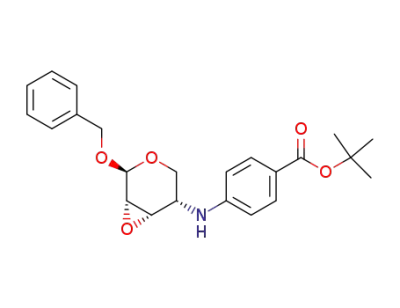
Benzyl 2,3-Anhydro-4-<4-(tert-butyloxycarbonyl)phenylamino>-4-deoxy-β-L-ribopyranoside
Relevant Products
-
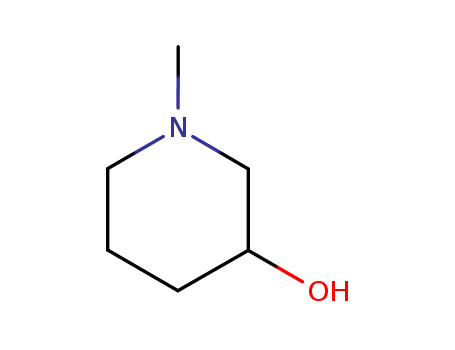
3-hydroxy-1-methylpiperidine
CAS:3554-74-3
-
Ammonium hydrogen fluoride
CAS:1341-49-7
-
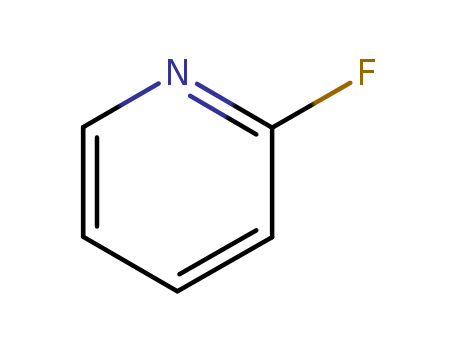
2-fluoropyridine
CAS:372-48-5