823-40-5
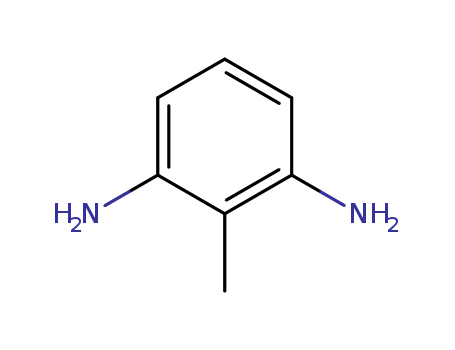
- Product Name:2,6-Diaminotoluene
- Molecular Formula:C7H10N2
- Purity:99%
- Molecular Weight:122.17
Product Details
Chemical plants supply high-quality 2,6-Diaminotoluene 823-40-5 in bulk
- Molecular Formula:C7H10N2
- Molecular Weight:122.17
- Appearance/Colour:off-white crystals
- Melting Point:104-106 °C(lit.)
- Refractive Index:1.5103 (estimate)
- Boiling Point:284.2 °C at 760 mmHg
- PKA:4.74±0.10(Predicted)
- Flash Point:148.3 °C
- PSA:52.04000
- Density:1.107 g/cm3
- LogP:2.32180
2,6-Diaminotoluene(Cas 823-40-5) Usage
|
Production Methods |
2,6-Toluenediamine is usually produced as a by-product with 2,4-TDA in mixtures containing 20% 2,6-isomer and 80% 2,4-isomer. It is used primarily in the manufacture of toluene diisocyanate, the predominant isocyanate in the flexible polyurethane foams and elastomers industry. Human exposure to 2,6-TDA may occur indirectly via exposure to toluene diisocyanate mixture containing 2,6- toluenediisocyanate, which is known to hydrolyze to 2,6- TDA rapidly upon contact with water. Workers in some plastics and elastomers industries may be exposed to atmosphere containing TDI. |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
2,6-Diaminotoluene neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Health Hazard |
ACUTE/CHRONIC HAZARDS: 2,6-Diaminotoluene is toxic. It is a local irritant. |
|
Fire Hazard |
Flash point data for 2,6-Diaminotoluene are not available. 2,6-Diaminotoluene is probably combustible. |
|
Definition |
ChEBI: A diamine that is toluene in which both of the hydrogens ortho- to the methyl group are replaced by amino groups. |
|
General Description |
Colorless prisms (from water). |
InChI:InChI=1/C7H10N2/c1-5-6(8)3-2-4-7(5)9/h2-4H,8-9H2,1H3
823-40-5 Relevant articles
Sustainable and recyclable palladium nanoparticles–catalyzed reduction of nitroaromatics in water/glycerol at room temperature
Chen, Jin,Dai, Bencai,Liu, Changchun,Shen, Zhihao,Zhao, Yongde,Zhou, Yang
, p. 540 - 544 (2020/07/14)
Palladium nanoparticles with unique cata...
N,S co-doped hierarchically porous carbon materials for efficient metal-free catalysis
Hu, Xiwei,Sun, Xun,Song, Qiang,Zhu, Yangyang,Long, Yu,Dong, Zhengping
, p. 742 - 752 (2020/02/21)
Metal-free carbon catalysts with excelle...
Co-MOF-Derived Hierarchical Mesoporous Yolk-shell-structured Nanoreactor for the Catalytic Reduction of Nitroarenes with Hydrazine Hydrate
Yuan, Man,Zhang, Hongbo,Yang, Chen,Wang, Fanhao,Dong, Zhengping
, p. 3327 - 3338 (2019/07/04)
Porous nanoreactors demonstrate immense ...
Pd-Pt/modified GO as an efficient and selective heterogeneous catalyst for the reduction of nitroaromatic compounds to amino aromatic compounds by the hydrogen source
Salahshournia, Hossein,Ghiaci, Mehran
, (2019/02/14)
In this work, different nitroaromatic co...
823-40-5 Process route
-
-
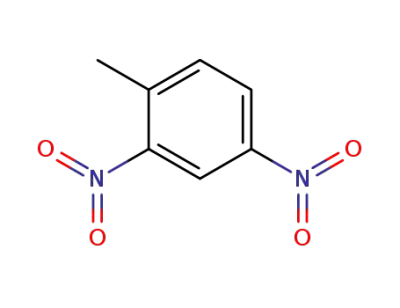
121-14-2
2,4-dinitrotoluene

-
-
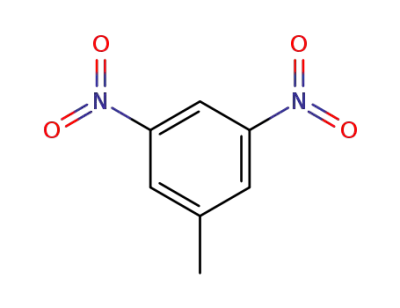
618-85-9
3,5-dinitrotoluene

-
-
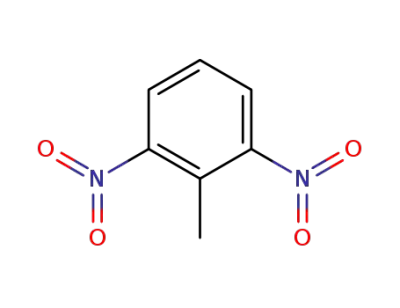
606-20-2
2,6-dinitrotoluene

-
-
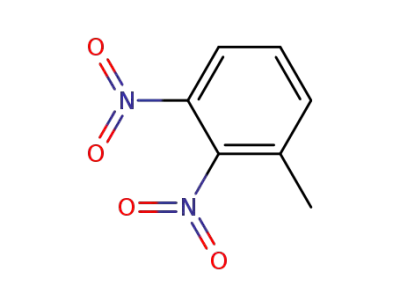
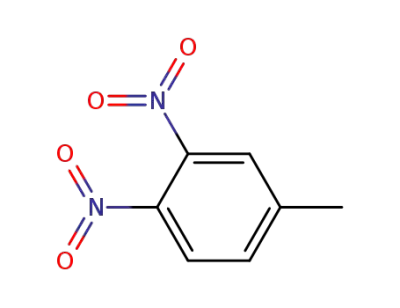
602-01-7
1-methyl-2,3-dinitrobenzene

-
-
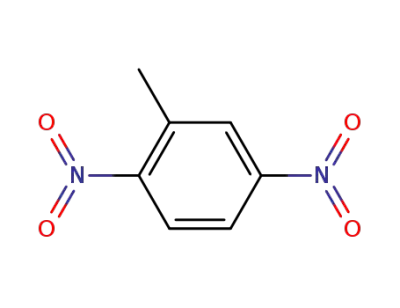
610-39-9
3,4-Dinitrotoluene

-
-
619-15-8
2,5-dinitrotoluene

-

-
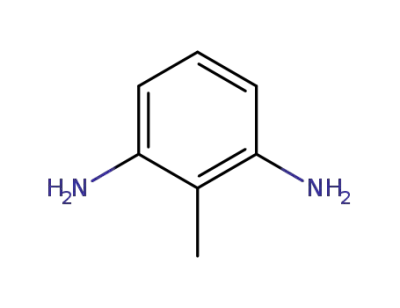
108-71-4
3,5-diaminotoluene

-
-
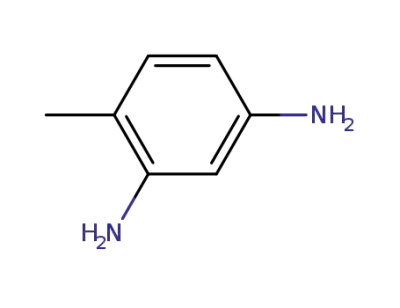
823-40-5
2,6-toluenediamine

-
-
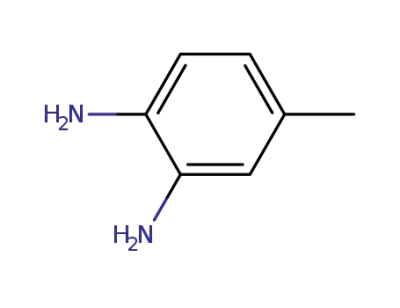
95-80-7,71111-07-4
4-methylbenzene-1,3-diamine

-
-
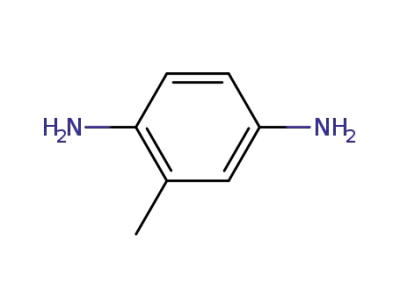
496-72-0
4-methyl-1,2-diaminobenzene

-
-
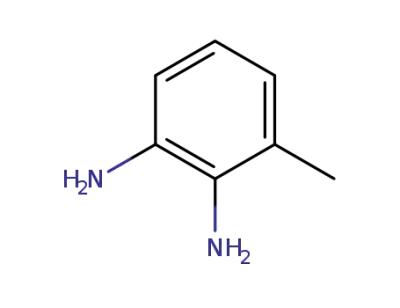
95-70-5
2-methyl-p-phenylenediamine

-
-
2687-25-4
3-methyl-1,2-benzenediamine
| Conditions | Yield |
|---|---|
|
With
hydrogen;
nickel;
at 120 - 140 ℃;
under 11251.1 - 22502.3 Torr;
Product distribution / selectivity;
|
-

-
606-20-2
2,6-dinitrotoluene

-

-
823-40-5
2,6-toluenediamine

-
-
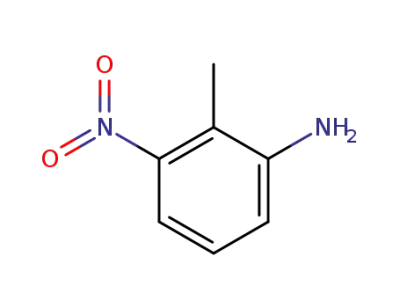
603-83-8
3-nitro-o-tolylamine
| Conditions | Yield |
|---|---|
|
In
phosphate buffer;
pH=2;
Electrochemical reaction;
|
823-40-5 Upstream products
-
606-20-2

2,6-dinitrotoluene
-
603-83-8

3-nitro-o-tolylamine
-
91-08-7
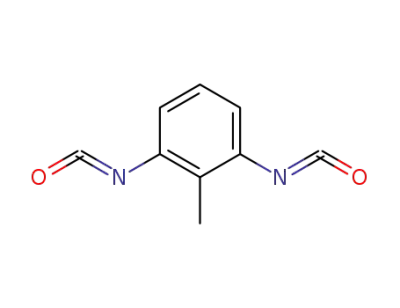
2,6-Toluene diisocyanate
-
7647-01-0
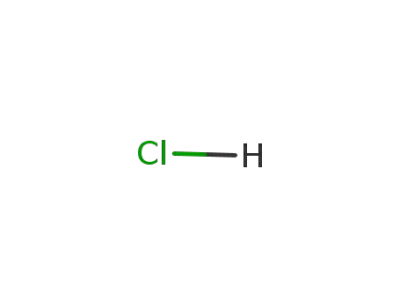
hydrogenchloride
823-40-5 Downstream products
-
58336-27-9
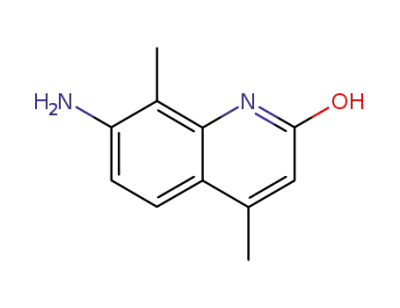
7-amino-4,8-dimethyl-quinolin-2-ol
-
91-08-7

2,6-Toluene diisocyanate
-
2095-01-4
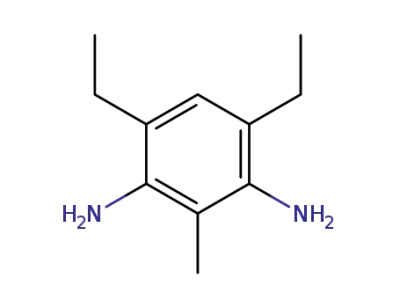
2-methyl-4,6-diethylphenylene-1,3-diamine
-
84434-42-4
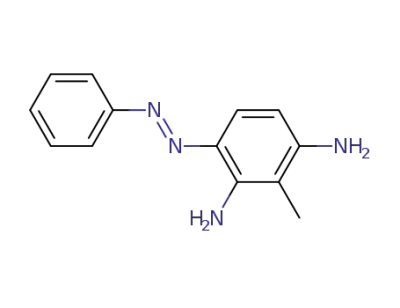
2-methyl-4-phenylazo-m-phenylenediamine
Relevant Products
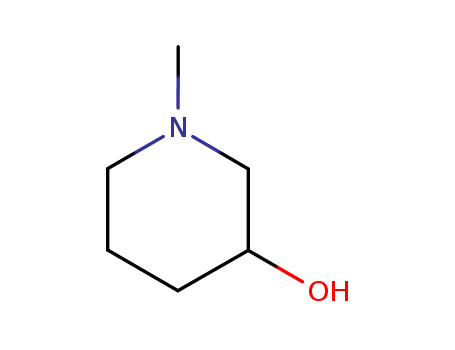
-
3-hydroxy-1-methylpiperidine
CAS:3554-74-3
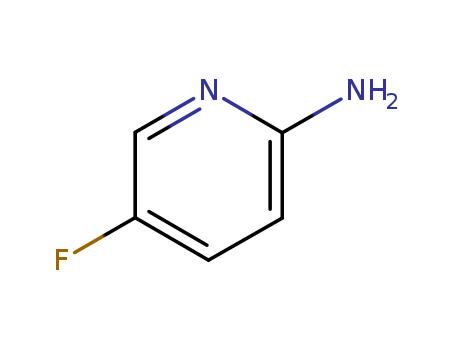
-
2-Amino-5-fluoropyridine
CAS:21717-96-4
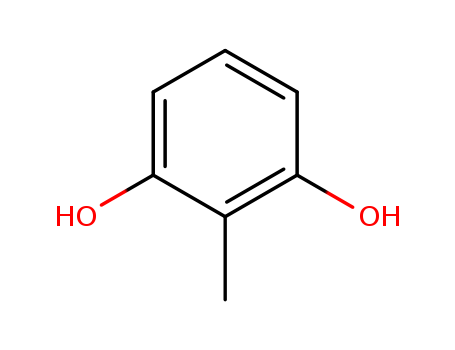
-
2-Methyl resorcinol
CAS:608-25-3