45644-21-1
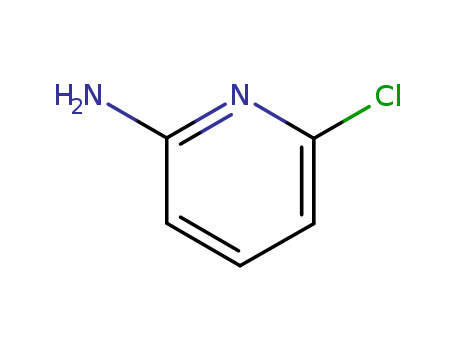
- Product Name:6-Chloro-2-pyridinamine
- Molecular Formula:C5H5ClN2
- Purity:99%
- Molecular Weight:128.561
Product Details
Cost-effective and customizable 6-Chloro-2-pyridinamine 45644-21-1 supplier
- Molecular Formula:C5H5ClN2
- Molecular Weight:128.561
- Appearance/Colour:White to light yellow powder
- Vapor Pressure:0.016mmHg at 25°C
- Melting Point:69-73 °C
- Refractive Index:1.607
- Boiling Point:255.72 °C at 760 mmHg
- PKA:2.76±0.24(Predicted)
- Flash Point:108.456 °C
- PSA:38.91000
- Density:1.326 g/cm3
- LogP:1.89840
2-Amino-6-chloropyridine(Cas 45644-21-1) Usage
InChI:InChI=1/C5H5ClN2/c6-4-2-1-3-5(7)8-4/h1-3H,(H2,7,8)
45644-21-1 Relevant articles
Protein kinase inhibitor and use thereof
-
Paragraph 0091; 0102-0103, (2021/01/21)
The present invention provides a compoun...
Preparation method of 2-amino substituted six-membered nitrogen-containing heterocycle complex
-
Paragraph 0025; 0026; 0079, (2019/02/08)
The invention discloses a preparation me...
Transition-metal-free access to 2-aminopyridine derivatives from 2-fluoropyridine and acetamidine hydrochloride
Li, Yibiao,Huang, Shuo,Liao, Chunshu,Shao, Yan,Chen, Lu
supporting information, p. 7564 - 7567 (2018/11/02)
Under catalyst-free conditions, an effic...
Catalytic application of 1,3,5-triazine-pentaethylenehexamine polymer-supported palladium nanoparticles in the convenient reduction of nitroarenes with sodium borohydride or hydrazine
Gen?, Hayriye,Zengin, Mustafa,Kü?ükislamo?lu, Mustafa,Imamoglu, Mustafa,Toplan, Hüseyin ?zkan,Arslan, Mustafa
, p. 784 - 792 (2017/11/20)
The catalytic activity of 1,3,5-triazine...
45644-21-1 Process route
-
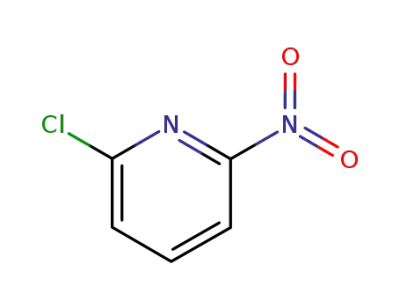
-
94166-64-0
2-nitro-6-chloro-pyridine

-
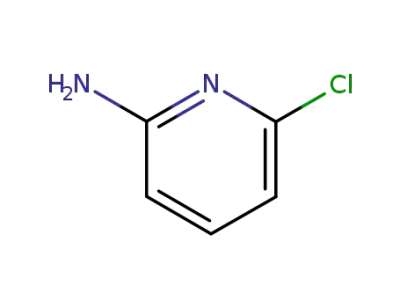
-
45644-21-1
6-chloropyridin-2-amine
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate;
In
ethanol; water;
at 20 ℃;
for 1.5h;
under 760.051 Torr;
Reagent/catalyst;
Solvent;
|
98% |
|
With
sodium tetrahydroborate;
In
ethanol; water;
at 20 ℃;
for 4h;
chemoselective reaction;
|
97% |
-
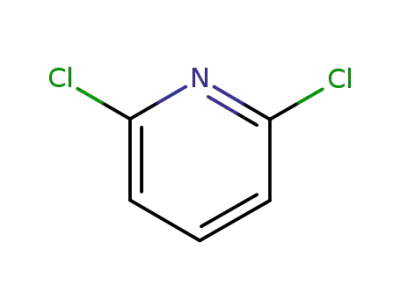
-
2402-78-0
2,6-dichloropyridine

-

-
45644-21-1
6-chloropyridin-2-amine
| Conditions | Yield |
|---|---|
|
With
ammonium hydroxide;
at 125 ℃;
for 5h;
under 1500.12 Torr;
|
79% |
|
With
ammonium hydroxide;
In
water;
at 180 ℃;
for 12h;
|
77% |
|
With
ammonia;
In
water;
at 150 ℃;
for 6h;
|
70% |
|
With
ammonium hydroxide;
at 180 ℃;
for 40h;
|
32% |
|
With
ammonia;
In
isopropyl alcohol;
at 160 ℃;
for 15h;
Microwave irradiation;
|
28% |
|
With
ammonium hydroxide;
at 180 - 190 ℃;
|
|
|
With
ammonium hydroxide;
In
ethanol;
|
|
|
With
ammonia;
In
ethanol;
at 160 - 165 ℃;
for 60h;
|
|
|
With
ammonium hydroxide;
In
ethanol;
|
45644-21-1 Upstream products
-
2402-78-0

2,6-dichloropyridine
-
98130-68-8
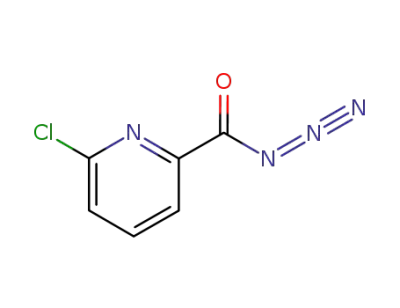
6-chloro-pyridine-2-carbonyl azide
-
13880-89-2
3-hydroxyglutaronitrile
-
80364-46-1
N-(6-chloropyridin-2-yl)acetamide
45644-21-1 Downstream products
-
17920-35-3
2-amino-6-methoxypyridine
-
86847-84-9
2,2-Dimethyl-N-(6-chloro-2-pyridinyl)propanamide
-
63763-86-0
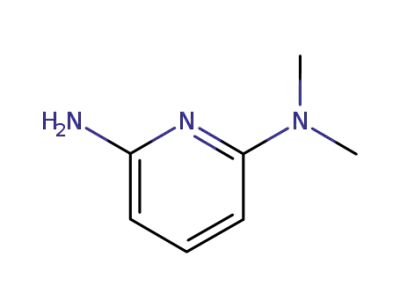
2-amino-6-N,N-dimethylamino pyridine
-
110270-89-8
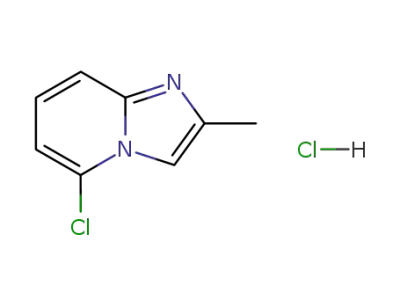
5-chloro-2-methylimidazo<1,2-a>pyridine hydrochloride
Relevant Products
-
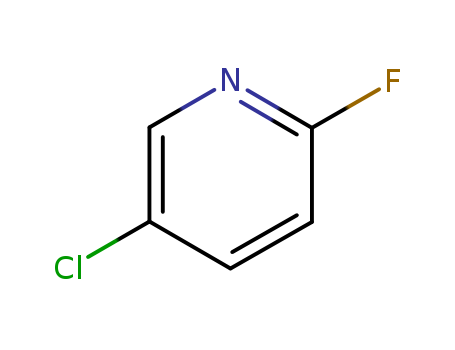
5-Chloro-2-fluoropyridine
CAS:1480-65-5
-
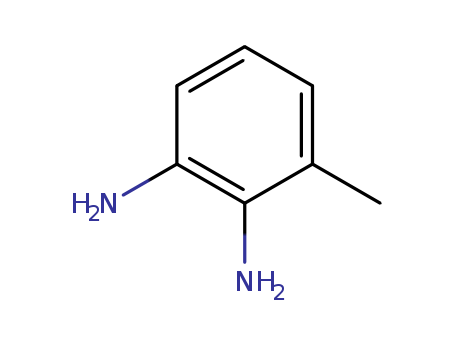
2,3-Diaminotoluene
CAS:2687-25-4
-
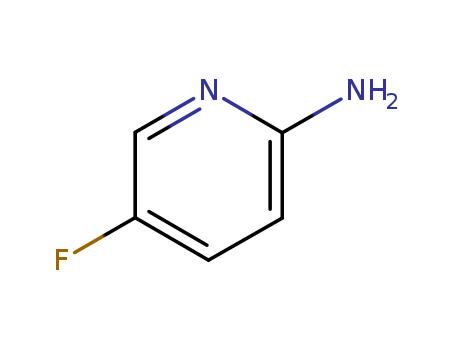
2-Amino-5-fluoropyridine
CAS:21717-96-4