3731-38-2
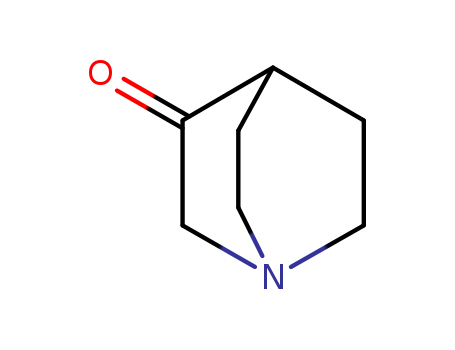
- Product Name:3-quinuclidone
- Molecular Formula:C7H11 N O
- Purity:99%
- Molecular Weight:125.17
Product Details
Buy cost-effective 99% pure 3-quinuclidone 3731-38-2 now
- Molecular Formula:C7H11 N O
- Molecular Weight:125.17
- Melting Point:200 °C
- Boiling Point:204.9°Cat760mmHg
- PKA:6.73±0.20(Predicted)
- Flash Point:78.1°C
- PSA:20.31000
- Density:1.12g/cm3
- LogP:0.21900
3-QUINUCLIDINONE(Cas 3731-38-2) Usage
InChI:InChI=1/C7H11NO/c9-7-5-8-3-1-6(7)2-4-8/h6H,1-5H2
3731-38-2 Relevant articles
New and potent quinuclidine-based antimicrobial agents
Kastelic, Andreja Radman,Od?ak, Renata,Pezdirc, Iskra,Sovi?, Karlo,Hrenar, Tomica,Ga?parovi?, Ana ?ipak,Sko?ibu?i?, Mirjana,Primo?i?, Ines
, (2019)
Developing new antibiotics is currently ...
Three new quinuclidine-based structures: Second harmonic generation response for 1,2-bis(1-azoniabicyclo[2.2.2]octan-3-ylidene)-hydrazine dichloride
Qiao, Liang,Chen, Xiao-Gang,Gao, Ji-Xing,Ai, Yong
, p. 728 - 733 (2019)
The crystal structures of three quinucli...
Directional Intermolecular Interactions for Precise Molecular Design of a High- Tc Multiaxial Molecular Ferroelectric
Yang, Chen-Kai,Chen, Wang-Nan,Ding, Yan-Ting,Wang, Jing,Rao, Yin,Liao, Wei-Qiang,Xie, Yongfa,Zou, Wennan,Xiong, Ren-Gen
, p. 1781 - 1787 (2019/01/26)
Quasi-spherical molecules have recently ...
Highly efficient and practical aerobic oxidation of alcohols by inorganic-ligand supported copper catalysis
Wei, Zheyu,Ru, Shi,Zhao, Qixin,Yu, Han,Zhang, Gang,Wei, Yongge
supporting information, p. 4069 - 4075 (2019/08/07)
The oxidation of alcohols to aldehydes o...
3731-38-2 Process route
-
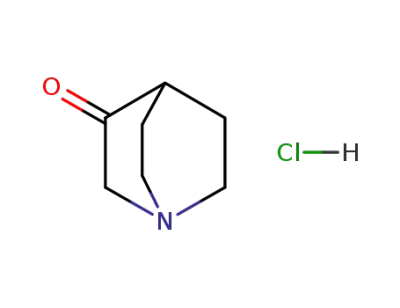
-
1193-65-3
3-quinuclidinone hydrochloride

-
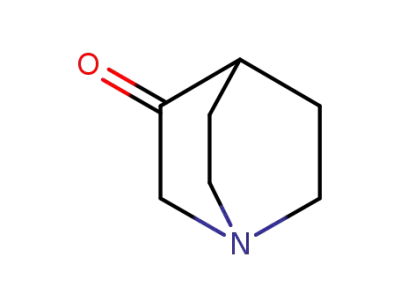
-
3731-38-2
3-Quinuclidinone
| Conditions | Yield |
|---|---|
|
With
sodium carbonate; sodium hydroxide;
In
water;
at 0 ℃;
for 0.5h;
|
100% |
|
With
sodium hydrogencarbonate;
In
water;
at 20 ℃;
for 0.166667h;
|
88% |
|
With
ammonia;
In
methanol;
|
|
|
With
water; sodium hydrogencarbonate;
|
|
|
With
sodium hydrogencarbonate;
In
water;
|
|
|
With
potassium carbonate;
In
dichloromethane;
for 0.5h;
|
|
|
With
sodium hydroxide;
|
|
|
With
sodium hydroxide;
In
water;
at 10 ℃;
Reagent/catalyst;
|
|
|
With
sodium hydroxide;
In
water;
|
|
|
With
potassium carbonate;
In
diethyl ether; water;
at 25 ℃;
for 0.5h;
|
-
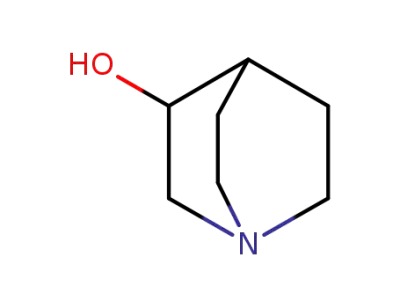
-
1619-34-7
3-quinuclidinol

-

-
3731-38-2
3-Quinuclidinone
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate;
C29H30ClNOP2Ru(1+);
In
acetone;
at 60 ℃;
for 10h;
Product distribution / selectivity;
Inert atmosphere;
|
95% |
|
With
(NH4)4[CuMo6O18(OH)6]·5H2O; oxygen; sodium sulfite;
In
water; acetonitrile;
at 60 ℃;
for 15h;
under 760.051 Torr;
|
95% |
|
With
dmap; [2,2]bipyridinyl; 2-azatricyclo[3.3.1.13,7]dec-2-yloxidanyl; copper(l) chloride;
In
acetonitrile;
at 20 ℃;
for 2h;
chemoselective reaction;
|
94% |
|
With
bromopentacarbonylmanganese(I); N-methyl-N,N-di(2-pyridylmethyl)amine; acetone; sodium t-butanolate;
In
toluene;
at 90 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
Darkness;
|
88% |
|
With
9-azabicyclo[3.3.1]nonan-3-one N-oxyl oxide; oxygen; nitric acid; sodium nitrite;
In
acetonitrile;
at 20 ℃;
for 6h;
|
54% |
|
With
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; triethylamine;
In
acetone;
at 150 ℃;
Flow reactor;
|
84 %Spectr. |
|
With
5H3N*5H(1+)*IMo6O24(5-); oxygen; sodium acetate;
In
water; acetonitrile;
at 70 ℃;
for 12h;
under 760.051 Torr;
Green chemistry;
|
89 %Chromat. |
3731-38-2 Upstream products
-
1838-39-7
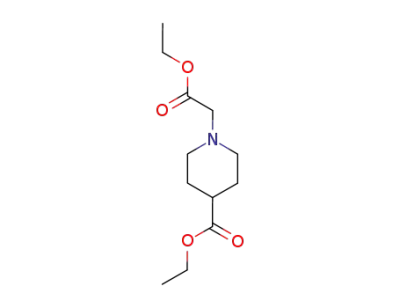
ethyl N-ethoxycarbonylmethyl-4-piperidinecarboxylate
-
100-76-5
Quinuclidine
-
161153-87-3
3-<(N-benzyl)acetamido>quinuclidine
-
3684-26-2
(S)-quinuclidin-3-ol
3731-38-2 Downstream products
-
16283-66-2
3-methylquinuclidin-3-ol
-
24123-89-5
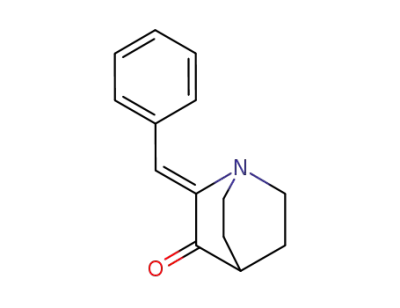
(Z)-2-benzylidene-1-azabicyclo[2.2.2]octan-3-one
-
42925-15-5
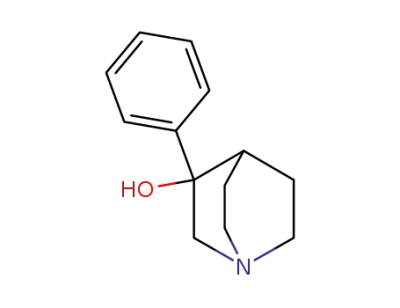
3-phenyl-quinuclidin-3-ol
-
111030-88-7
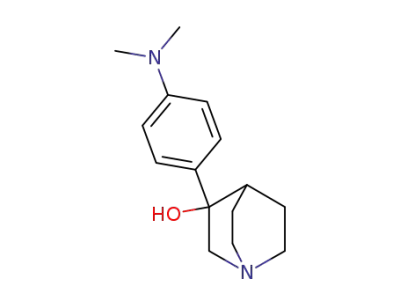
3-(4-dimethylamino-phenyl)-quinuclidin-3-ol
Relevant Products
-
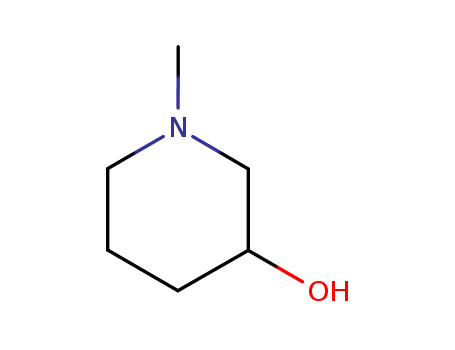
3-hydroxy-1-methylpiperidine
CAS:3554-74-3
-
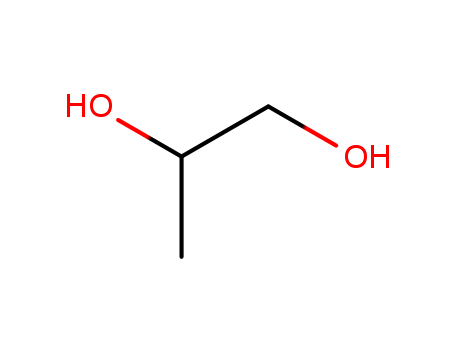
Propylene Glycol
CAS:57-55-6
-
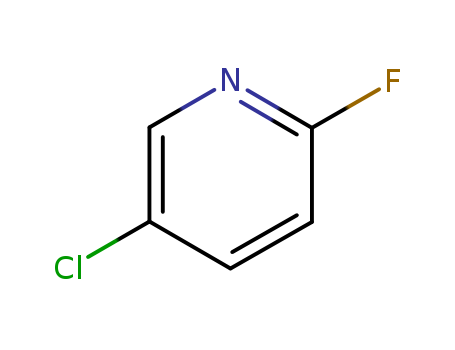
5-Chloro-2-fluoropyridine
CAS:1480-65-5