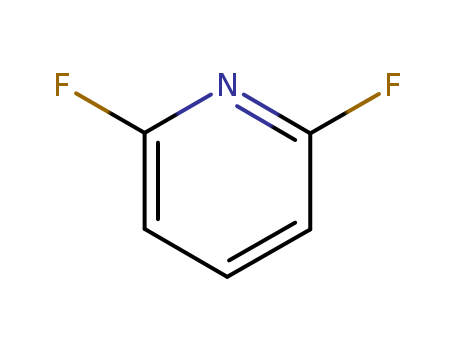
1513-65-1
- Product Name:2,6-Difluoropyridine
- Molecular Formula:C5H3F2N
- Purity:99%
- Molecular Weight:115.082
Product Details
Manufacturers supply cost-effective and customizable 2,6-Difluoropyridine 1513-65-1
- Molecular Formula:C5H3F2N
- Molecular Weight:115.082
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:0.643mmHg at 25°C
- Refractive Index:n20/D 1.437(lit.)
- Boiling Point:134.3 °C at 760 mmHg
- PKA:-6.09±0.10(Predicted)
- Flash Point:33.9 °C
- PSA:12.89000
- Density:1.263 g/cm3
- LogP:1.35980
2,6-Difluoropyridine(Cas 1513-65-1) Usage
|
General Description |
Lithium diisopropylamide (LDA)-mediated ortholithiations of 2,6-difluoropyridine in tetrahydrofuran at -78°C has been studied using a combination of IR and NMR spectroscopic and computational methods. Polycondensation of bistrimethylsilyl derivatives of various diphenols with 2,6-difluoropyridine in N-methylpyrrolidone in the presence of K2CO3 has been investigated. |
InChI:InChI=1/C5H4F3N3O/c6-5(7,8)2-1-3(12)11-4(9)10-2/h1H,(H3,9,10,11,12)
1513-65-1 Relevant articles
Highly reactive and regenerable fluorinating agent for oxidative fluorination of aromatics
Janmanchi, Krishna Murthy,Dolbier Jr., William R.
, p. 349 - 354 (2008)
A newly synthesized copper aluminum fluo...
NEW DEVELOPMENTS IN THE SYNTHESIS OF LOWER FLUORINATED PYRIDINES VIA DIAZOTIZATION-FLUORINATION OF AMINOPYRIDINES IN ANHYDROUS HYDROGEN FLUORIDE
Boudakian, Max M.
, p. 497 - 506 (1981)
The isolation and stabilization of elusi...
-
Lui et al.
, p. 583 (1978)
-
Method for efficiently synthesizing fluorine-containing compound
-
Paragraph 0074-0076, (2021/06/26)
The invention discloses a method for eff...
PROCESS FOR FLUORINATING COMPOUNDS
-
Page/Page column 29; 33; 35, (2017/02/28)
Disclosed are mild temperature (e.g., fr...
Rhodium catalyzed, carbon-hydrogen bond directed hydrodefluorination of fluoroarenes
Ekkert, Olga,Strudley, Sebastian D. A.,Rozenfeld, Alisa,White, Andrew J. P.,Crimmin, Mark R.
supporting information, p. 7027 - 7030 (2015/05/19)
[CpRhCl(μ-Cl)]2 is reported as a highly ...
Acyl azolium fluorides for room temperature nucleophilic aromatic fluorination of chloro- and nitroarenes
Ryan, Sarah J.,Schimler, Sydonie D.,Bland, Douglas C.,Sanford, Melanie S.
supporting information, p. 1866 - 1869 (2015/04/27)
The reaction of acid fluorides with N-he...
1513-65-1 Process route
-
-
110-86-1
pyridine

-
-
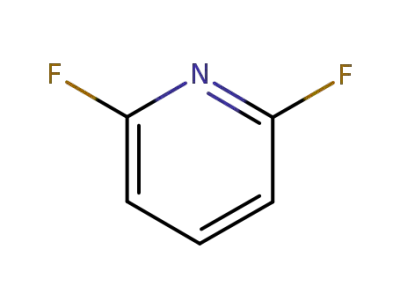
1513-65-1
2,6-difluoro pyridine

-
-
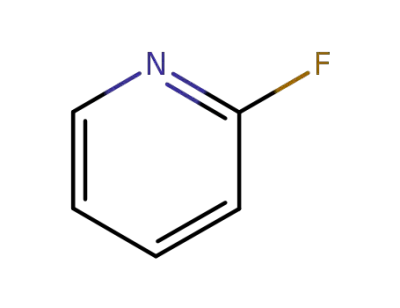
372-48-5
2-fluoropyridine
| Conditions | Yield |
|---|---|
|
With
co-precipitated copper aluminum fluoride (CuAl01);
at 500 ℃;
Gas phase;
Inert atmosphere;
|
32% 11% |
|
With
Al2CuF8;
at 500 ℃;
Reagent/catalyst;
Inert atmosphere;
|
32% 11% |
|
With
iodine; fluorine;
In
1,1,2-Trichloro-1,2,2-trifluoroethane;
at 0 ℃;
Yield given;
Yields of byproduct given;
|
|
|
With
iodine; fluorine;
In
1,1,2-Trichloro-1,2,2-trifluoroethane;
at 0 ℃;
Yields of byproduct given;
|
3 % Chromat. |
-

-
110-86-1
pyridine

-
-
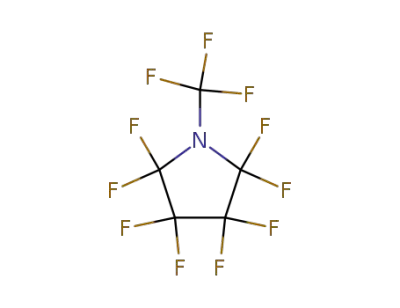
2344-10-7
perfluoro-N-ethylpyrrolidine

-

-
1513-65-1
2,6-difluoro pyridine

-
-
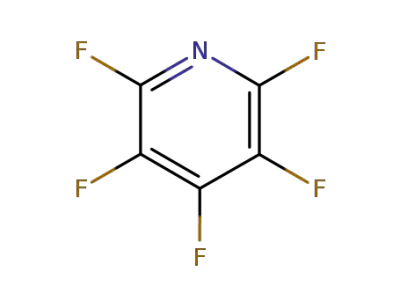
700-16-3
Pentafluoropyridine

-
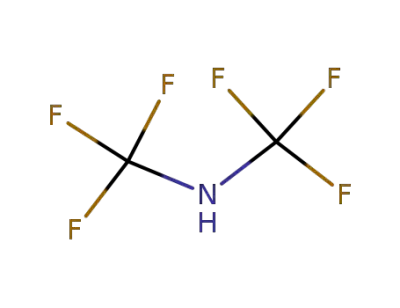
-
371-77-7
N,N-Bis(trifluoromethyl)amine
| Conditions | Yield |
|---|---|
|
With
caesium tetrafluorocobaltate(III);
In
gaseous matrix;
at 310 ℃;
for 3.5h;
Further byproducts given;
|
35.3% 13.6% 8.2% 1.6% |
1513-65-1 Upstream products
-
110-86-1

pyridine
-
109-09-1
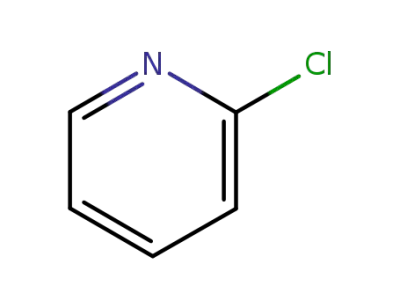
2-chloropyridine
-
141-86-6
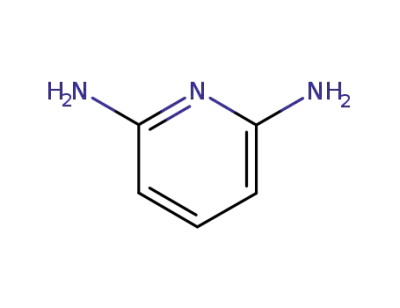
2.6-diaminopyridine
-
119071-51-1
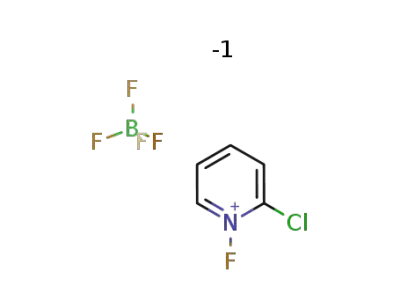
C5H4ClFN(1+)*BF4(1-)
1513-65-1 Downstream products
-
858675-60-2
2-ethoxy-6-fluoropyridine
-
133635-20-8
(3aR,4R,6R,6aR)-4-(2,6-Difluoro-pyridin-3-yl)-2,2-dimethyl-6-trityloxymethyl-tetrahydro-furo[3,4-d][1,3]dioxol-4-ol
-
58602-02-1
2,6-Difluoro-3-nitropyridine
-
171178-50-0
2,6-difluoronicotinic acid
Relevant Products
-
5-Chloro-2-fluoropyridine
CAS:1480-65-5
-
2-Fluoroisonicotinic acid
CAS:402-63-5
-
4-Methoxybenzoic acid
CAS:100-09-4