1670-14-0
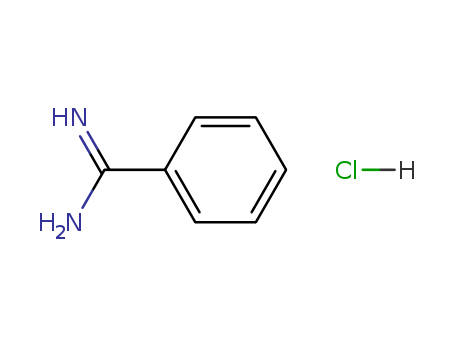
- Product Name:Benzamidine hydrochloride
- Molecular Formula:C7H8N2.HCl
- Purity:99%
- Molecular Weight:156.615
Product Details
Good factory supply good Benzamidine hydrochloride 1670-14-0
- Molecular Formula:C7H8N2.HCl
- Molecular Weight:156.615
- Appearance/Colour:white to off-white powder
- Vapor Pressure:5.63E-05mmHg at 25°C
- Melting Point:86-88 °C(lit.)
- Boiling Point:208.5 °C at 760 mmHg
- Flash Point:79.9 °C
- PSA:49.87000
- Density:1.09 g/cm3
- LogP:2.57270
Benzamidine hydrochloride(Cas 1670-14-0) Usage
|
Preparation |
To a stainless steel rocking autoclave equipped with a stirrer is added 103.0 gm (1.0 mole) of benzonitrile, 214.0 gm (4.0 mole) of ammonium chloride, and 306.0 gm (18.0 mole) of ammonia is introduced by means of a transfer bomb. The reaction mixture is heated at 150°C for 18 hr (pressure: 1300-6500 psig), cooled, and, with appropriate precautions for the safe control of the excess ammonia, is vented to atmospheric pressure. The reaction mixture is extracted with ether to remove approximately 5% unreacted benzonitrile, and then extracted with hot acetonitrile or ethanol to separate the amidine hydrochloride from the unreacted ammonium chloride. Concentration of the latter affords 120.5 gm (77%) of benzamidine hydrochloride, m.p. 161-163°C. |
|
Biological Activity |
Benzamidine hydrochloride is an reversible competitive inhibitor of trypsin-like serine proteases, with Kis of 97 μM, 21 μM, 20 μM and 110 μM for uPA, trypsin, tryptase and factor Xa, respectively. |
|
Safety Profile |
Moderately toxic byintraperitoneal route. When heated to decomposition itemits toxic vapors of NOx, HCl, and Cl-. |
|
in vitro |
Benzamidine hydrochloride has weak inhibition for tPA and thrombin, with Kis of 750 μM and 320 μM, respectively. |
InChI:InChI=1/C7H8N2.ClH.H2O/c8-7(9)6-4-2-1-3-5-6;;/h1-5H,(H3,8,9);1H;1H2
1670-14-0 Relevant articles
Ethylene bis-imidazoles are highly potent and selective activators for isozymes VA and VII of carbonic anhydrase, with a potential nootropic effect
Draghici, Bogdan,Vullo, Daniela,Akocak, Suleyman,Walker, Ellen A.,Supuran, Claudiu T.,Ilies, Marc A.
, p. 5980 - 5983 (2014)
A series of ethylene bis-imidazoles was ...
Synthesis, Molecular Structure and Solution Dynamics of Dimeric Benzamidinates containing a Double Diazaallyl Lithium Bridge. A Rapid Interconversion of ? and ? Bonds
Eisen, Moris S.,Kapon, Moshe
, p. 3507 - 3510 (1994)
Dimeric Li reacted with the nitriles 4-X...
2-methoxyphenoxy pyrimidine antitumor compound as well as preparation method and application thereof
-
Paragraph 0045; 0050-0051, (2021/05/12)
The invention belongs to the technical f...
Phenyl pyrimidinamine anti-tumor compound as well as preparation method and application thereof
-
Paragraph 0045; 0050-0051, (2021/06/06)
The invention belongs to the technical f...
Synthesis method of benzamidine hydrochloride
-
Paragraph 0023; 0025; 0026; 0027; 0030; 0031, (2017/12/09)
The invention discloses a synthesis meth...
'Green' synthesis of 2-substituted 6-hydroxy-[3H]-pyrimidin-4-ones and 4,6-dichloropyrimidines: Improved strategies and mechanistic study
Opitz, Andreas,Sulger, Werner,Daltrozzo, Ewald,Koch, Rainer
, p. 814 - 824 (2015/05/20)
An improved route to 2-substituted 6-hyd...
1670-14-0 Process route
-
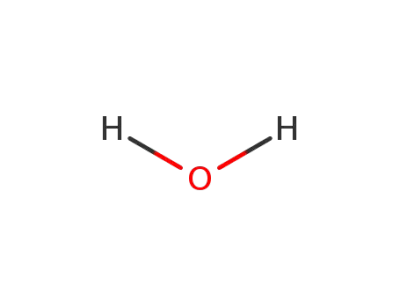
-
7732-18-5
water

-
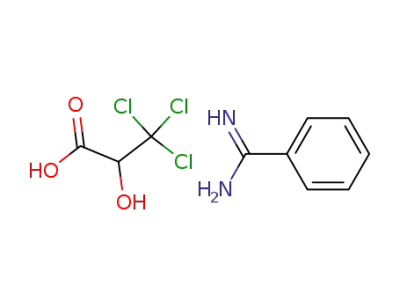
-
benzamidine; trichlorolactate

-
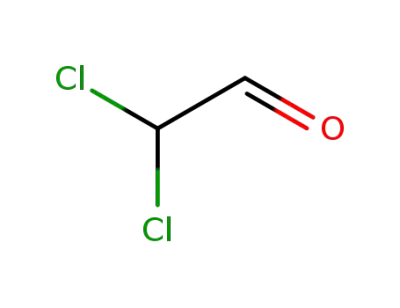
-
79-02-7
2,2-dichloroacetaldehyde

-
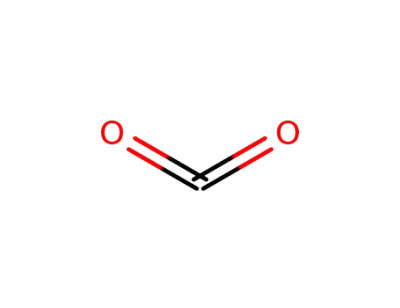
-
124-38-9,18923-20-1
carbon dioxide

-
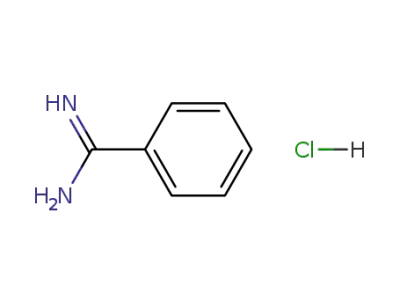
-
1670-14-0
benzamidine monohydrochloride
| Conditions | Yield |
|---|---|
|
|
-
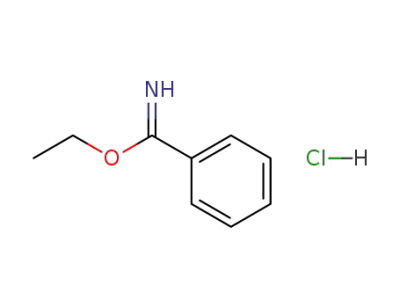
-
5333-86-8
ethyl benzimidate hydrochloride

-

-
1670-14-0
benzamidine monohydrochloride
| Conditions | Yield |
|---|---|
|
With
ammonium hydroxide;
In
ethanol;
at 20 ℃;
for 24h;
|
80% |
|
With
ammonia;
In
ethanol;
for 5h;
Green chemistry;
|
1670-14-0 Upstream products
-
100-47-0

benzonitrile
-
7664-41-7
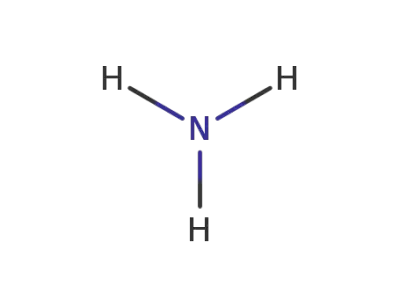
ammonia
-
5333-86-8

ethyl benzimidate hydrochloride
-
7732-18-5

water
1670-14-0 Downstream products
-
100869-87-2
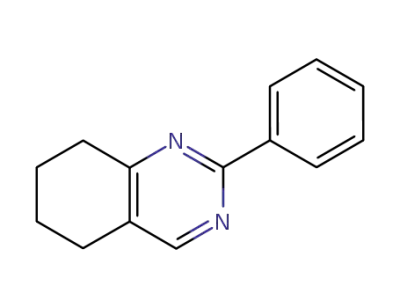
2-phenyl-5,6,7,8-tetrahydroquinazoline
-
68906-00-3
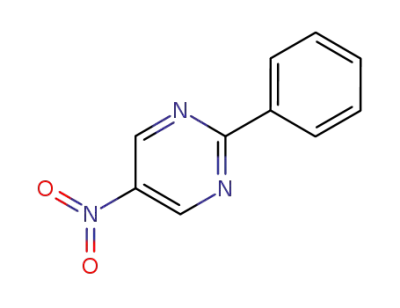
2-phenyl-5-nitropyrimidine
-
1722-18-5
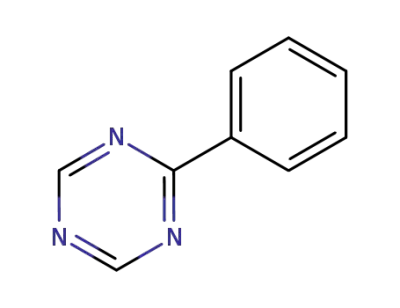
2-phenyl-1,3,5-triazine
-
86739-33-5
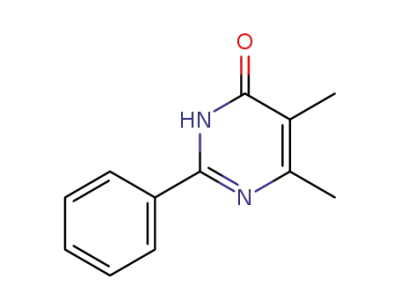
5,6-dimethyl-2-phenylpyrimidin-4(3H)-one
Relevant Products
-
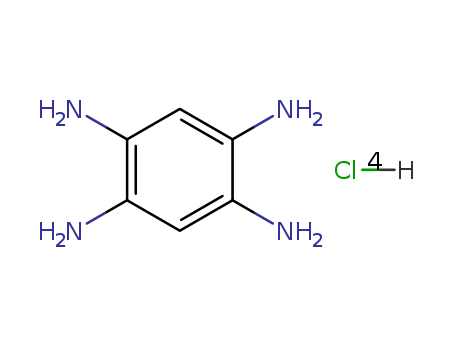
1,2,4,5-Benzenetetramine tetrahydrochloride
CAS:4506-66-5
-
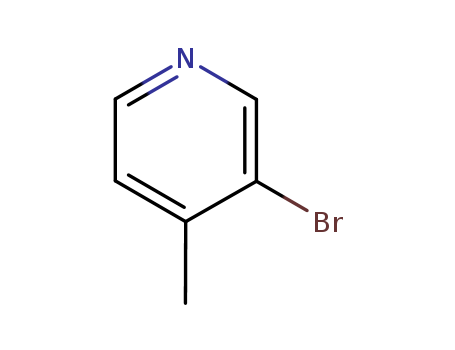
3-Bromo-4-Methylpyridine
CAS:3430-22-6
-
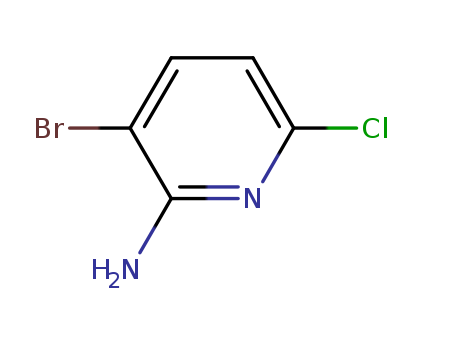
3-bromo-6-chloropyridin-2-amine
CAS:442127-50-6