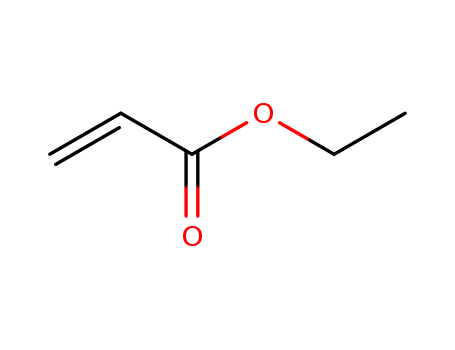
140-88-5
- Product Name:Ethyl Acrylate
- Molecular Formula:C5H8O2
- Purity:99%
- Molecular Weight:100.117
Product Details
High quality purity >99% Ethyl Acrylate 140-88-5 for sale
- Molecular Formula:C5H8O2
- Molecular Weight:100.117
- Appearance/Colour:clear colorless liquid
- Vapor Pressure:31 mm Hg ( 20 °C)
- Melting Point:-71 °C(lit.)
- Refractive Index:n20/D 1.406(lit.)
- Boiling Point:99.499 °C at 760 mmHg
- Flash Point:15.556 °C
- PSA:26.30000
- Density:0.913 g/cm3
- LogP:0.73550
Ethyl acrylate(Cas 140-88-5) Usage
|
Chemical Description |
Ethyl acrylate, ethyl crotonate, and diethyl fumarate are all esters that can be used in similar reactions. |
|
Preparation |
By esterification of acrylic acid; by heating acetylene with HCl in alcoholic solution in the presence of Ni(CO)4; also from ethyl-3-chloropropionate passed over activated carbon at high temperature. |
|
Production Methods |
Ethyl acrylate is manufactured via oxidation of propylene to acrolein and then to acrylic acid. The acid is treated with ethanol to yield the ethyl ester . Vinyl chloride reacts at 270 °C at >6895 kPa (68 atm) with ethanol in the presence of a cobalt and palladium catalyst to give ethyl acrylate in a yield of 17% . |
|
Air & Water Reactions |
Highly flammable. Insoluble in water. |
|
Reactivity Profile |
A flammable liquid, confirmed carcinogen. Ethyl acrylate can react vigorously with oxidizing reagents, peroxides,strong alkalis and polymerization initiators. [NTP] Ethyl acrylate reacts violently with chlorosulfonic acid [Sax, 9th ed., 1996, p. 1515]. When an inhibited monomer was placed in a clear glass bottle exposed to sunlight, exothermic polymerization set in and caused the bottle to burst. The use of brown glass or metal containers and increase in inhibitor concentration (to 200 ppm; tenfold) was recommended [MCA Case History No. 1759]. Ethyl acrylate may polymerize when exposed to light and Ethyl acrylate is subject to slow hydrolysis. Inhibitors do not function in the absence of air. Solutions in DMSO are stable for 24 hours under normal lab conditions. [NTP]. |
|
Hazard |
Toxic by ingestion, inhalation, skin absorption; irritant to skin and eyes. Flammable, dangerous fire and explosion hazard. Possible carcinogen. |
|
Health Hazard |
Ethyl acrylate is a strong irritant to the eyes,skin, and mucous membranes. The liquid orits concentrated solutions can produce skinsensitization upon contact. It is toxic by allroutes of exposure. The toxicity is low inrats and mice and moderate in rabbits. Thetoxic effects from inhalation noted in animalswere congestion of lungs and degenerativechanges in the heart, liver, and kidney. Mon key exposed to 272 ppm for 28 days showedlethargy and weight loss; while exposure to1024 ppm caused death to the animals after2.2 days (Treon et al. 1949). By compari son, guinea pigs died of exposure to about1200 ppm for 7 hours. Ingestion of the liq uid may result in irritation of gastrointestinaltracts, nausea, lethargy, and convulsionsThe LD50 values varied significantly indifferent species of animals. The oral LD50values in rabbits, rats, and mice are in therange 400, 800, and 1800 mg/kg, respectively. Animals administered ethyl acrylateshowed increased incidence of tumors inforestomach. However, there is no evidenceof carcinogenicity caused by Ethyl acrylatein humans. |
|
Flammability and Explosibility |
Flammable |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: May occur; exclude moisture, light; avoid exposure to high temperatures; store in presence of air; Inhibitor of Polymerization: 13-17 ppm monomethyl ether of hydroquinone. |
|
Safety Profile |
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion and inhalation. Moderately toxic by skin contact and intraperitoneal routes. Human systemic effects by inhalation: eye, olfactory, and pulmonary changes. A skin and eye irritant. Characterized in its terminal stages by dyspnea, cyanosis, and convulsive movements. It caused severe local irritation of the gastroenteric tract; and toxic degenerative changes of cardiac, hepatic, renal, and splenic tissues were observed. It gave no evidence of cumulative effects. When applied to the intact skin of rabbits, the ethyl ester caused marked local irritation, erythema, edema, thickening, and vascular damage. Animals subjected to a fairly high concentration of these esters suffered irritation of the mucous membranes of the eyes, nose, and mouth as well as lethargy, dpspnea, and convulsive movements. A substance that migrates to food from packagmg materials. Flammable liquid. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Violent reaction with chlorosulfonic acid. To fight fire, use CO2, dry chemical, or alcohol foam. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS. |
|
Safety |
It is an acute toxin with an LD50 (rats, oral) of 1020 mg / kg and a TLV of 5 ppm. The International Agency for Research on Cancer stated, "Overall evaluation, Ethyl acrylate is possibly carcinogenic to humans (Group 2B)." The United States Environmental Protection Agency (EPA) states, "Human studies on occupational exposure to ethyl acrylate... have suggested a relationship between exposure to the chemical(s) and colorectal cancer, but the evidence is conflicting and inconclusive. In a study by the National Toxicology Program (NTP), increased incidences of squamous cell papillomas and carcinomas of the fore stomach were observed in rats and mice exposed via gavage (experimentally placing the chemical in the stomach). However, the NTP recently determined that these data were not relevant to human carcinogenicity since humans do not have a fore stomach, and removed ethyl acrylate from its list of carcinogens." (Occupational exposure generally involves exposure that occurs regularly, over an extended period of time.) One favorable safety aspect is that ethyl acrylate has good warning properties; the odor threshold is much lower than any level of health concern. In other words, the bad odor warns people of ethyl acrylate's presence long before the concentration reaches a level capable of creating a serious health risk. |
|
Potential Exposure |
This material is used in emulsion polymers for paints, textiles, adhesives, coatings and binders; as a monomer in the manufacture of homopolymer and copolymer resins for the production of paints and plastic films |
|
Carcinogenicity |
A retrospective study found an excess of colorectal cancers in one exposed population of workers; however, the data were confounded by other exposures and lack of association of causality and risk in similarly exposed populations from other locations. Therefore, there was inadequate evidence based on the study that ethyl acrylate is a human carcinogen . Ethyl acrylate is listed as USEPA group B2, “Probable human carcinogen”; IARC group B2, “Possibly carcinogenic in humans”; NIOSH, “Carcinogen with no further categorization”; NTP group 2, “Reasonably anticipated to be a carcinogen” and listed as a carcinogen by California Proposition 65 . Dermal studies of acrylic acid, ethyl acrylate, and n-butyl acrylate using mice did not result in local carcinogenesis, but several mice in the ethyl acrylate-treated group did exhibit dermatitis, dermal fibrosis, epidermal necrosis, and hyperkeratosis . |
|
Environmental fate |
Chemical/Physical. Polymerizes on standing and is catalyzed by heat, light, and peroxides (Windholz et al., 1983). Slowly hydrolyzes in water forming ethanol and acrylic acid. The reported rate constant for the reaction of ethyl acrylate with ozone in the gas phase was determined to be 5.70 x 10-18 cm3 mol/sec (Munshi et al., 1989). At an influent concentration of 1,015 mg/L, treatment with GAC resulted in an effluent concentration of 226 mg/L. The adsorbability of the carbon used was 157 mg/g carbon (Guisti et al., 1974). |
|
Shipping |
UN1917 Ethyl acrylate, Hazard Class: 3; Labels: 3-Flammable liquid |
|
Purification Methods |
Wash the ester repeatedly with aqueous NaOH until free from inhibitors such as hydroquinone, then wash it with saturated aqueous CaCl2 and distil it under reduced pressure. Hydroquinone should be added if the ethyl acrylate is to be stored for extended periods. [Beilstein 2 IV 1460.] LACHRYMATORY. |
|
Toxicity evaluation |
The toxic mode of action for ethyl acrylate is unknown. However, the parent compound may play a significant role since pretreatment of rats with a carboxylesterase inhibitor enhances the respiratory irritation and lethality produced by the inhalation of ethyl acrylate. The enhanced toxicity could be a direct effect of methyl acrylate on surrounding tissues and/or a secondary effect due to the increased conjugation of methyl acrylate with glutathione that occurs under these conditions which in turn can result in toxicity due to the depletion of local glutathione stores. |
|
Incompatibilities |
May form explosive mixture with air. Atmospheric moisture and strong alkalies may cause fire and explosions unless properly inhibited (Note: Inert gas blanket not recommended). Heat, light or peroxides can cause polymerization. Incompatible with oxidizers (may be violent), peroxides, polymerizers, strong alkalis; moisture, chlorosulfonic acid, strong acids; amines. May accumulate static electrical charges, and may cause ignition of its vapors. Polymerizes readily unless an inhibitor, such as hydroquinone is added. Uninhibited vapors may plug vents by the formation of polymers. |
|
Waste Disposal |
Incineration or by absorption and landfill disposal |
|
General Description |
Ethyl acrylate is an acrylic ester derived from the esterification of acrylic acid with ethanol, often catalyzed by homogeneous or heterogeneous catalysts like sulfuric acid or ion-exchange resins. It can also be produced via the cleavage of nickelalactones using alkyl iodides, such as C2H5I, in a process involving Ni─O ring opening and β-H elimination. Additionally, ethyl acrylate may be indirectly obtained through the catalytic conversion of methyl lactate to acrylic acid and its subsequent esterification. Ethyl acrylate is significant in industrial applications, particularly in polymer production, and its synthesis can be optimized through various catalytic pathways to improve yield and selectivity. |
|
Physical properties |
Clear, colorless liquid with a penetrating and pungent odor. Leonardos et al. (1969) and Nagata and Takeuchi (1990) reported odor threshold concentrations of 0.47 and 0.26 ppbv, respectively. Experimentally determined detection and recognition odor threshold concentrations were 1.0 μg/m3 (0.24 ppbv) and 1.5 μg/m3 (0.37 ppbv), respectively (Hellman and Small, 1974). |
|
Aroma threshold values |
Detection: 0.2 ppb |
InChI:InChI=1/C5H8O2/c1-3-4(2)5(6)7/h2-3H2,1H3,(H,6,7)/p-1
140-88-5 Relevant articles
Asymmetric Counter-Anion-Directed Aminomethylation: Synthesis of Chiral β-Amino Acids via Trapping of an Enol Intermediate
Kang, Zhenghui,Wang, Yongheng,Zhang, Dan,Wu, Ruibo,Xu, Xinfang,Hu, Wenhao
, p. 1473 - 1478 (2019)
A novel enantioselective aminomethylatio...
Liberation of acrylates from nickelalactones via Ni─O ring opening with alkyl iodides
Zhang, Zhizhi,Guo, Fangjie,Kühn, Fritz E.,Sun, Jing,Zhou, Mingdong,Fang, Xiangchen
, (2017)
The utilization of carbon dioxide as a f...
Homogeneous and Heterogeneous Catalyzed Esterification of Acrylic Acid with Ethanol: Reaction Kinetics and Modeling
Jyoti, Ghoshna,Keshav, Amit,Anandkumar,Bhoi, Stutee
, p. 370 - 380 (2018)
Kinetics of esterification of acrylic ac...
-
Strohmeier,Weigelt
, p. C40 (1977)
-
Elucidating colorization in the functionalization of hydroxyl-containing polymers using unsaturated anhydrides/acyl chlorides in the presence of triethylamine
Cai, Lei,Wang, Shanfeng
, p. 304 - 307 (2010)
-
-
Garner et al.
, p. 532,535 (1959)
-
Efficient and selective conversion of methyl lactate to acrylic acid using Ca3(PO4)2-Ca2(P2O 7) composite catalysts
Hong, Ju Hyeong,Lee, Jong-Min,Kim, Hyungrok,Hwang, Young Kyu,Chang, Jong-San,Halligudi, Shiva B.,Han, Yo-Han
, p. 194 - 200 (2011)
Calcium phosphate Ca3(PO4)2 and calcium ...
WITTIG-HORNER REACTION CATALYZED BY ACTIVATED BARIUM HYDROXIDE IN THE PRESENCE OF ULTRASOUND
Fuentes, A.,Marinas, J.M.,Sinisterra, J.V.
, p. 2951 - 2954 (1987)
The sonochemical Wittig-Horner reaction,...
Controlling the Lewis Acidity and Polymerizing Effectively Prevent Frustrated Lewis Pairs from Deactivation in the Hydrogenation of Terminal Alkynes
Geng, Jiao,Hu, Xingbang,Liu, Qiang,Wu, Youting,Yang, Liu,Yao, Chenfei
, p. 3685 - 3690 (2021/05/31)
Two strategies were reported to prevent ...
Acid- And base-switched palladium-catalyzed γ-C(sp3)-H alkylation and alkenylation of neopentylamine
Zhang, Jinquan,Zhang, Shuaizhong,Zou, Hongbin
supporting information, p. 3466 - 3471 (2021/05/31)
The functionalization of remote unactiva...
Phosphine-Catalyzed Cascade Annulation of MBH Carbonates and Diazenes: Synthesis of Hexahydrocyclopenta[c]pyrazole Derivatives
Guo, Hongchao,Li, Hongxiang,Liu, Hao,Shi, Wangyu,Wang, Chang,Wang, Wei,Wu, Yongjun
supporting information, p. 5571 - 5575 (2021/07/31)
A phosphine-catalyzed cascade annulation...
METHOD FOR PRODUCING α,β-UNSATURATED CARBOXYLIC ACID DERIVATIVE
-
Paragraph 0102; 0108; 0113; 0115, (2021/04/02)
To provide a method for producing α,β-un...
140-88-5 Process route
-
-
106-98-9,9003-28-5
1-butylene

-
-
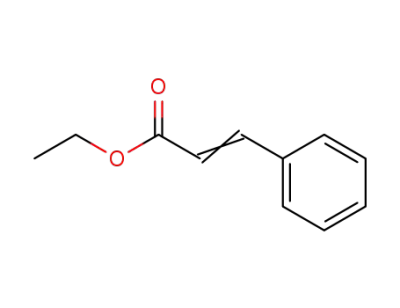
103-36-6
ethyl 3-phenyl-2-propenoate

-
-
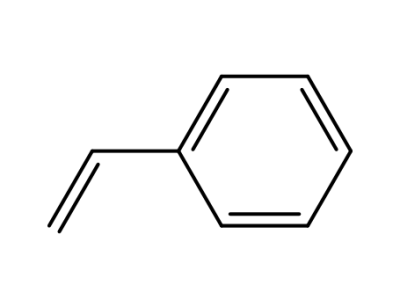
100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-
-
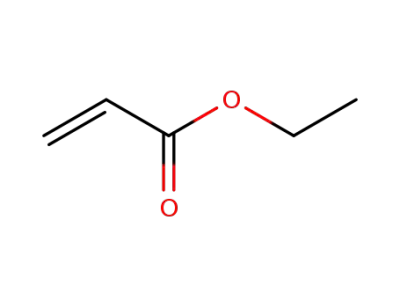
140-88-5,9003-32-1
ethyl acrylate
| Conditions | Yield |
|---|---|
|
Hoveyda-Grubbs catalyst second generation;
In
dichloromethane;
at 40 ℃;
for 24h;
under 750.075 Torr;
Inert atmosphere;
|
8% 8% |
-

-
103-36-6
ethyl 3-phenyl-2-propenoate

-
-
74-85-1
ethene

-

-
100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-

-
140-88-5,9003-32-1
ethyl acrylate
| Conditions | Yield |
|---|---|
|
Hoveyda-Grubbs catalyst second generation;
In
dichloromethane;
at 40 ℃;
for 24h;
under 562.556 Torr;
Product distribution / selectivity;
Inert atmosphere;
|
28% 19% |
|
With
Hoveyda-Grubbs catalyst second generation;
at 25 ℃;
for 21h;
under 13187.6 Torr;
Reagent/catalyst;
Catalytic behavior;
|
6.2% |
140-88-5 Upstream products
-
64-18-6
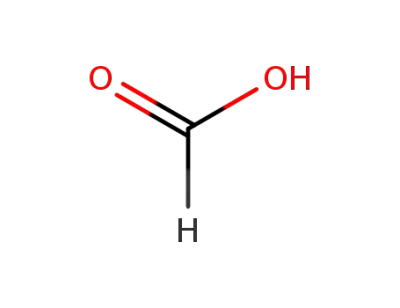
formic acid
-
590-29-4
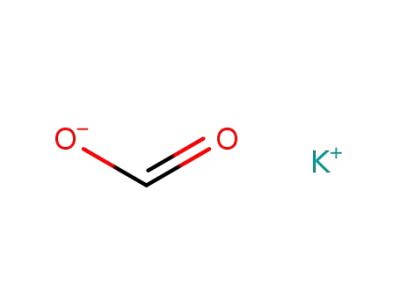
potassium formate
-
86931-46-6
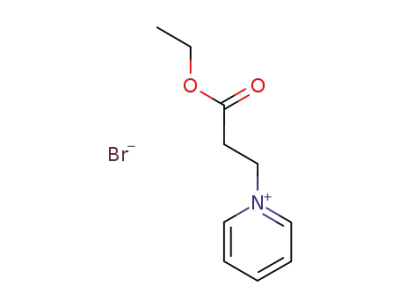
1-(2-carbethoxyethyl)pyridinium bromide
-
97-64-3
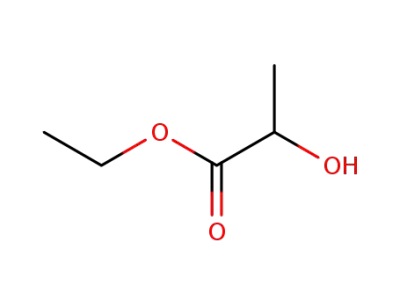
ethyl 2-hydroxypropionate
140-88-5 Downstream products
-
4078-22-2
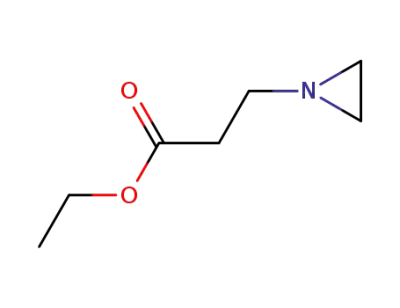
3-Aziridino-propionsaeure-ethylester
-
6317-35-7
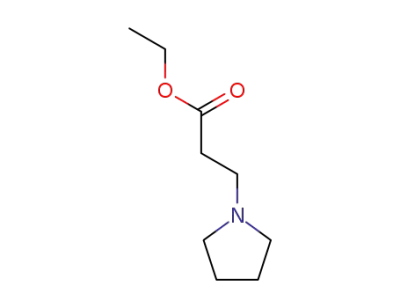
ethyl 3-tetrahydro-1H-1-pyrrolilpropanoate
-
19653-33-9
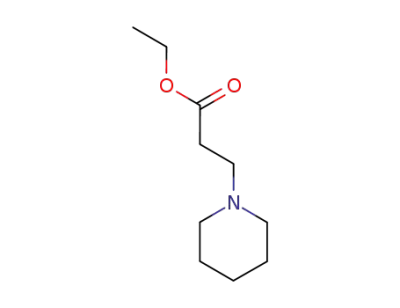
ethyl piperidine-1-propionate
-
20120-24-5
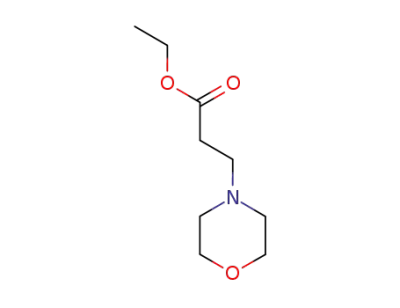
ethyl 3-morpholinopropionate
Relevant Products
-
Bismuth Octoate
CAS:67874-71-9
-
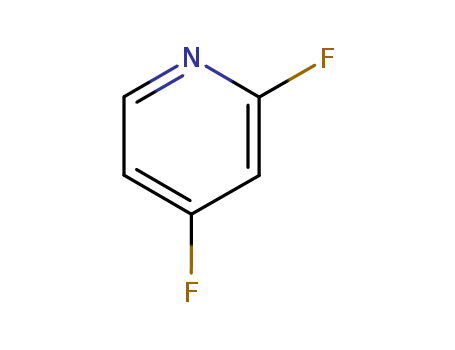
2,4-Difluoropyridine
CAS:34941-90-7
-
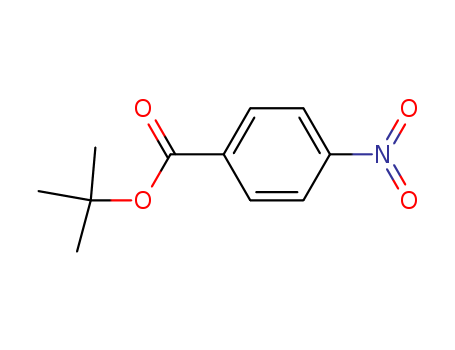
tert-Butyl 4-nitrobenzoate
CAS:19756-72-0