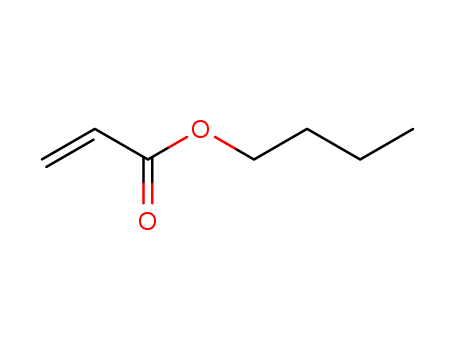
141-32-2
- Product Name:Butyl Acrylate
- Molecular Formula:C7H12O2
- Purity:99%
- Molecular Weight:128.171
Product Details
Bulk supply high purity Butyl Acrylate 141-32-2, Paid sample available
- Molecular Formula:C7H12O2
- Molecular Weight:128.171
- Appearance/Colour:clear colorless Liquid
- Vapor Pressure:0.039mmHg at 25°C
- Melting Point:-69 °C
- Refractive Index:n20/D 1.410(lit.)
- Boiling Point:145.9 °C at 760 mmHg
- Flash Point:39.4 °C
- PSA:26.30000
- Density:0.898 g/cm3
- LogP:1.51570
n-Butyl acrylate(Cas 141-32-2) Usage
|
General Description |
n-Butyl acrylate is a chemical compound belonging to the family of acrylate monomers, commonly used in the production of polymers and coatings. It is a clear, flammable liquid with a fruity odor and is highly reactive with a potential for polymerization if not properly stored and handled. n-Butyl acrylate is primarily used in the production of adhesives, sealants, and coatings for various applications such as paints, varnishes, and inks. It is also utilized in the manufacture of textiles, paper, and leather products and as a co-monomer in the synthesis of copolymers to enhance the properties of the final material. However, exposure to n-Butyl acrylate can cause irritations to the skin, eyes, and respiratory system, and long-term exposure may have harmful effects on human health. Therefore, it should be handled with care and appropriate safety measures should be in place when working with this chemical. |
InChI:InChI=1/C7H12O2/c1-3-4-5-6(2)7(8)9/h2-5H2,1H3,(H,8,9)/p-1
141-32-2 Relevant articles
Kinetics of the synthesis of propyl and butyl acrylates in the presence of some heteropolyacids as catalysts
Skrzypek, Ierzy,Witczak, Teresa,Grzesik, MirosLaw,Witczak, Mariusz
, p. 12 - 17 (2009)
Esterification reactions of acrylic acid...
Electrocatalytic Oxidation of Allylic Ethers, Dihydropyran and Phenol Using a Polypyridyl Complex of Ruthenium(IV)
Campos, Jose Luiz,Giovani, Wagner F. De,Romero, Jose Ricardo
, p. 597 - 599 (1990)
An electrocatalytic procedure is describ...
Silica-Supported zirconium complexes and their polyoligosilsesquioxane analogues in the transesterification of acrylates: Part 2. activity, recycling and regeneration
Salinier, Valerie,Niccolai, Geraldp,Dufaud, Veronique,Basset, Jean-Marie
, p. 2168 - 2177 (2009)
The catalytic activity of both supported...
Acrylate Esters by Ethenolysis of Maleate Esters with Ru Metathesis Catalysts: an HTE and a Technoeconomic Study
Copéret, Christophe,De Jesus Silva, Jordan,Engl, Pascal S.,Fedorov, Alexey,Lange, Jean-Paul,Togni, Antonio,Tsygankov, Alexey
, (2020)
A high throughput experimentation (HTE) ...
SYNTHESIS OF ACRYLIC ESTERS BY LIPASE
Ikeda, Isao,Tanaka, Jun,Suzuki, Kimihiro
, p. 6865 - 6866 (1991)
Various acrylic esters were synthesized ...
Acid- And base-switched palladium-catalyzed γ-C(sp3)-H alkylation and alkenylation of neopentylamine
Zhang, Jinquan,Zhang, Shuaizhong,Zou, Hongbin
supporting information, p. 3466 - 3471 (2021/05/31)
The functionalization of remote unactiva...
Palladium-catalyzed remote C-H functionalization of 2-aminopyrimidines
Das, Animesh,Jana, Akash,Maji, Biplab
supporting information, p. 4284 - 4287 (2020/04/27)
A straightforward strategy was developed...
Synthesis of Some Aromatic and Aliphatic Esters Using WO3/ZrO2 Solid Acid Catalyst under Solvent Free Conditions
Guguloth, Vijaya Charan,Battu, Satyanarayana
, p. 2153 - 2157 (2020/09/16)
A simple method is delineated for the sy...
Second-Generation meta-Phenolsulfonic Acid-Formaldehyde Resin as a Catalyst for Continuous-Flow Esterification
Hu, Hao,Ota, Hajime,Baek, Heeyoel,Shinohara, Kenta,Mase, Toshiaki,Uozumi, Yasuhiro,Yamada, Yoichi M. A.
supporting information, p. 160 - 163 (2020/01/02)
A second-generation m-phenolsulfonic aci...
141-32-2 Process route
-
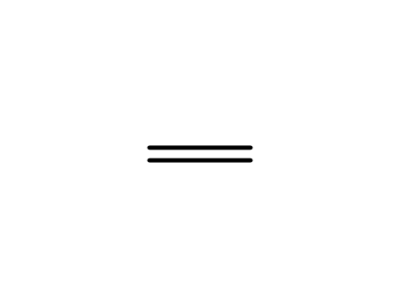
-
74-85-1
ethene

-
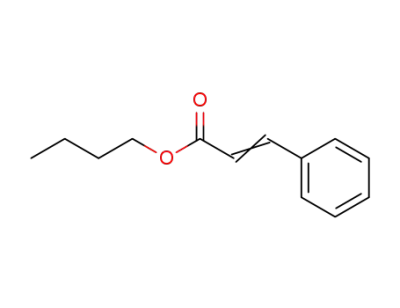
-
52392-64-0,52392-65-1,538-65-8
n-butyl cinnamate

-
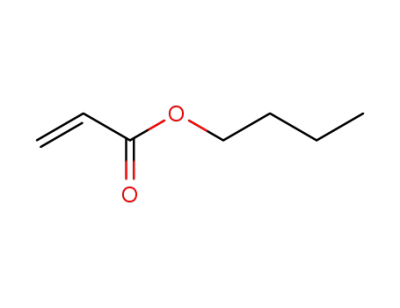
-
141-32-2,9003-49-0
acrylic acid n-butyl ester

-
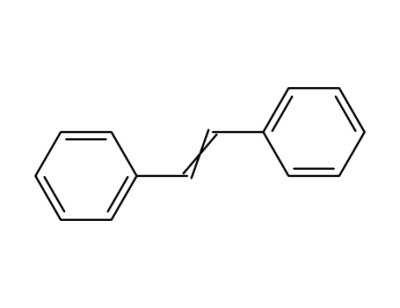
-
588-59-0
stilbene
| Conditions | Yield |
|---|---|
|
With
Hoveyda-Grubbs catalyst second generation;
In
dichloromethane;
at 40 ℃;
for 24h;
under 750.075 Torr;
Pressure;
Concentration;
Inert atmosphere;
Green chemistry;
|
-
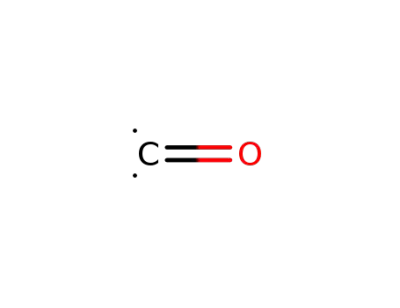
-
201230-82-2
carbon monoxide

-
-
74-86-2,25067-58-7
acetylene

-
-
71-36-3
butan-1-ol

-
-
590-01-2
propionic acid butyl ester

-

-
141-32-2,9003-49-0
acrylic acid n-butyl ester

-
-
141-03-7
dibutyl succinate
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
dicarbonyl(η5-cyclopentadienyl)nitrosylchromium;
at 70 ℃;
under 750.06 Torr;
Rate constant;
other catalysts;
|
141-32-2 Upstream products
-
463-51-4
Ketene
-
50-00-0
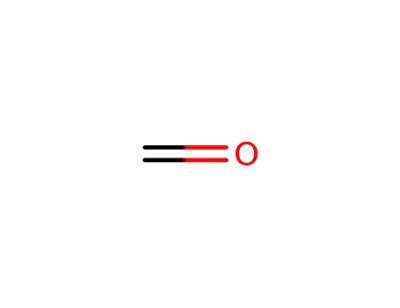
formaldehyd
-
123-86-4
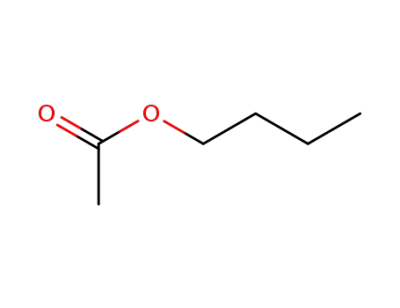
acetic acid butyl ester
-
5422-69-5
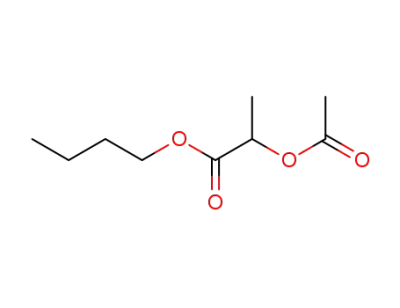
butyl 2-acetoxypropionic acid
141-32-2 Downstream products
-
108903-76-0
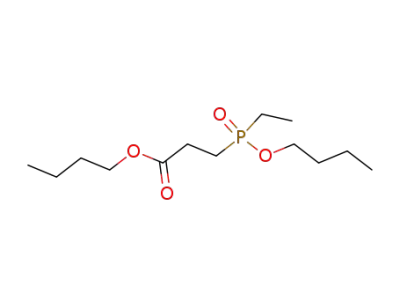
butyl 3-(ethylbutoxyphosphinyl)propionate
-
14144-48-0
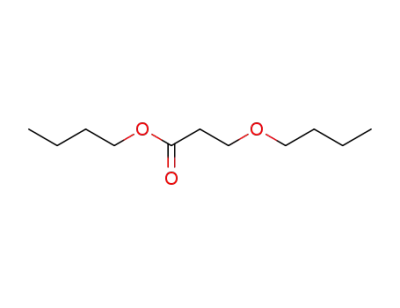
butyl 3-butoxypropionate
-
27387-79-7
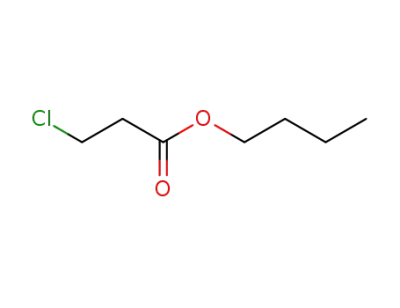
3-chloro-propionic acid butyl ester
-
6973-79-1
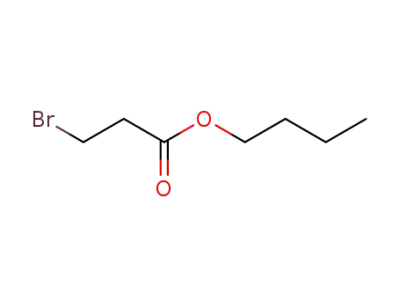
butyl 3-bromopropanate
Relevant Products
-
Bismuth Octoate
CAS:67874-71-9
-
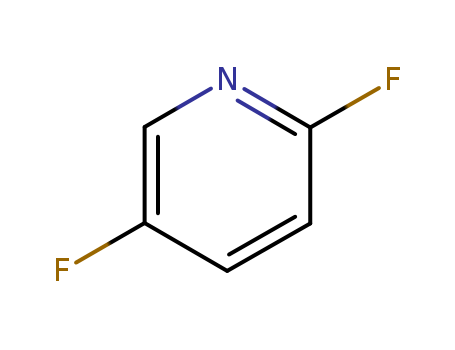
2,5-Difluoropyridine
CAS:84476-99-3
-
Ethanediammonium Diacetate
CAS:38734-69-9