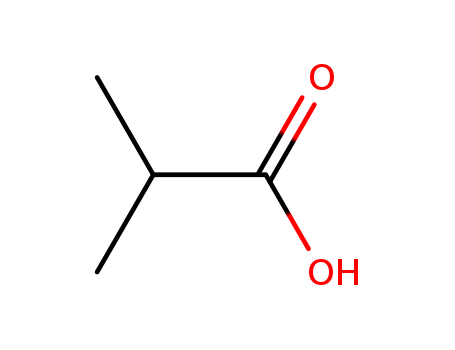
79-31-2
- Product Name:Isobutyric Acid
- Molecular Formula:C4H8O2
- Purity:99%
- Molecular Weight:88.1063
Product Details
Cost-effective and customizable Isobutyric Acid 79-31-2 in stock
- Molecular Formula:C4H8O2
- Molecular Weight:88.1063
- Appearance/Colour:clear colorless liquid
- Vapor Pressure:1.5 mm Hg ( 20 °C)
- Melting Point:-47 °C
- Refractive Index:n20/D 1.393(lit.)
- Boiling Point:155.2 °C at 760 mmHg
- PKA:4.84(at 20℃)
- Flash Point:58.2 °C
- PSA:37.30000
- Density:0.983 g/cm3
- LogP:0.72700
Isobutyric acid(Cas 79-31-2) Usage
|
Physical properties |
Isobutyric Acid is a flavoring agent that is a colorless liquid with a strong, penetrating odor, resembling butter. it is miscible in alcohol, propylene glycol, glycerin, mineral oil, and most fixed oils and soluble in water. it is obtained by chemical synthesis. it is also termed isopropylformic acid. |
|
Preparation |
Isobutyric acid is prepared in a similar way to butyric acid, mainly by direct oxidation of isobutanol and isobutyraldehyde, which is obtained by a direct oxidation reaction with air or oxygen. |
|
Application |
Isobutyric acid is mainly used in the synthesis of isobutyric acid esters, such as methyl isobutyrate, propyl ester, isoamyl ester and benzyl ester. It can also be used manufacture of esters for solvents, flavors and perfume bases, disinfecting agent, varnish, plasticizers, deliming hides, tanning agent and used in pharmaceutical. Isobutyric acid has some important derivatives that, in the industry, is actually used for the production of isobutyronitrile intermediates, and then converted to isobutylamidine hydrochloride that is the raw materials of pesticide diazinon. |
|
Definition |
ChEBI: Isobutyric acid is a branched fatty acid comprising propanoic acid carrying a methyl branch at C-2. It has a role as a volatile oil component, a plant metabolite and a Daphnia magna metabolite. It is a branched-chain saturated fatty acid, a methyl-branched fatty acid and a fatty acid 4:0. It is a conjugate acid of an isobutyrate. |
|
Aroma threshold values |
Detection: 10 ppb to 9.5 ppm; aroma characteristics at 10 ppm: acidic pungent, dairy buttery and cheesy with fruity undertones. |
|
Taste threshold values |
Taste characteristics at 15 ppm: acidic, sour dairy, creamy, cheese, cultured dairy nuance. |
|
General Description |
Isobutyric acid appears as a colorless liquid with a light odor of rancid butter. Flash point 132°F. Density 7.9 lb / gal. Corrosive to metals and tissue. |
|
Air & Water Reactions |
Flammable. Water soluble |
|
Reactivity Profile |
Isobutyric acid corrodes aluminum and other metals. Flammable hydrogen gas may accumulate in enclosed spaces in which this reaction has taken place [USCG, 1999]. |
|
Health Hazard |
Inhalation causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Contact with eyes or skin causes irritation. |
|
Fire Hazard |
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
|
Biochem/physiol Actions |
Odor at 10 ppm |
|
Synthesis |
The preparation of isobutyric acid is similar with butyric acid, which is performed by the direct oxidation of isobutyl alcohol and isobutyraldehyde. Isobutyric acid can be directly generated from the oxidation of isobutyraldehyde in air or oxygen. Other manufacturing methods have isobutyronitrile hydrolysis and methacrylic acid hydrogenation. The oxidation of 2-methyl-1-nitropropane to prepare isobutyric acid can also obtain a higher yield. The purification of Isobutyric acid can be realized by azeotropic distillation with water, and anhydrous isobutyric acid can be obtained by the extractive distillation from carbon tetrachloride. Propylene and formic acid ester can react at 50 °C with the catalysis of hydrofluoric acid to generate methyl isobutyrate and propyl isobutyrate. |
|
Purification Methods |
Distil the acid from KMnO4, then redistil it from P2O5. [Beilstein 2 H 288, 2 I 126, 2 II 257, 2 III 637, 2 IV 843.] |
InChI:InChI=1/2C4H8O2/c2*1-3(2)4(5)6/h2*3H,1-2H3,(H,5,6)
79-31-2 Relevant articles
-
Simons,Werner
, p. 1356 (1942)
-
(Hexamethylbenzene)Ru catalysts for the Aldehyde-Water Shift reaction
Phearman, Alexander S.,Moore, Jewelianna M.,Bhagwandin, Dayanni D.,Goldberg, Jonathan M.,Heinekey, D. Michael,Goldberg, Karen I.
supporting information, p. 1609 - 1615 (2021/03/09)
The Aldehyde-Water Shift (AWS) reaction ...
Time-Dependent Self-Assembly of Copper(II) Coordination Polymers and Tetranuclear Rings: Catalysts for Oxidative Functionalization of Saturated Hydrocarbons
Costa, Ines F. M.,Kirillova, Marina V.,André, Vania,Fernandes, Tiago A.,Kirillov, Alexander M.
supporting information, p. 14491 - 14503 (2021/07/19)
This study describes a time-dependent se...
A 3D MOF based on Adamantoid Tetracopper(II) and Aminophosphine Oxide Cages: Structural Features and Magnetic and Catalytic Properties
?liwa, Ewelina I.,Nesterov, Dmytro S.,Kirillova, Marina V.,K?ak, Julia,Kirillov, Alexander M.,Smoleński, Piotr
supporting information, p. 9631 - 9644 (2021/06/30)
This work describes an unexpected genera...
Aqueous Persistent Noncovalent Ion-Pair Cooperative Coupling in a Ruthenium Cobaltabis(dicarbollide) System as a Highly Efficient Photoredox Oxidation Catalyst
Guerrero, Isabel,Vi?as, Clara,Fontrodona, Xavier,Romero, Isabel,Teixidor, Francesc
, p. 8898 - 8907 (2021/06/28)
An original cooperative photoredox catal...
79-31-2 Process route
-
-
C36H60O30*C4H8O2

-
-
79-31-2
isobutyric Acid

-
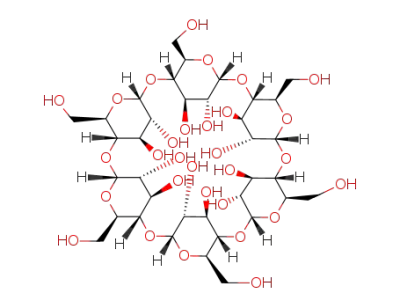
-
10016-20-3
alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
With
phosphate buffer;
In
water-d2;
at 25 ℃;
Equilibrium constant;
Thermodynamic data;
standard molar enthalpy ΔrH0;
|
-
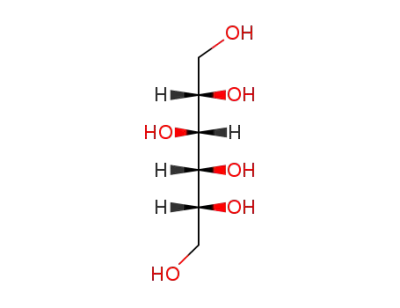
-
50-70-4
D-sorbitol

-
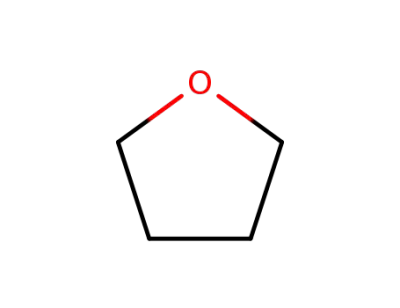
-
109-99-9,24979-97-3,77392-70-2
tetrahydrofuran

-
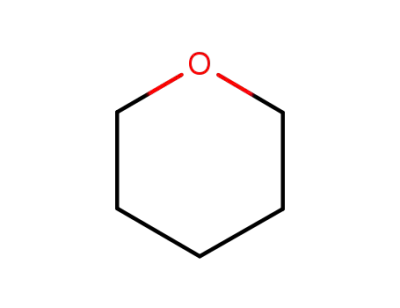
-
142-68-7
TETRAHYDROPYRANE

-

-
96-47-9
2-methyltetrahydrofuran

-
-
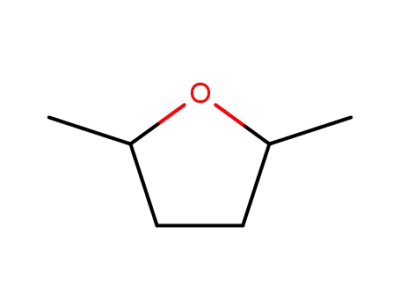
1003-38-9
2,5-dimethyltetrahydrofuran

-
-
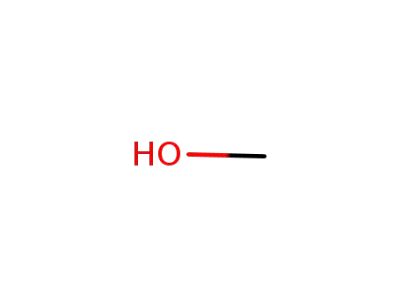
67-56-1
methanol

-
-
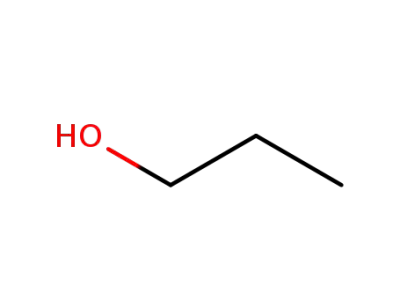
71-23-8
propan-1-ol

-
-
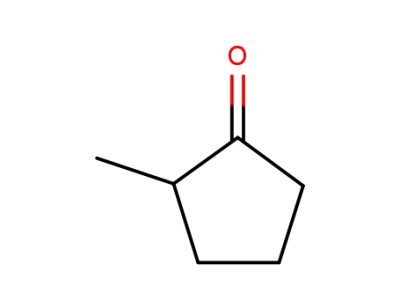
1120-72-5
2-Methylcyclopentanone

-
-
1757-42-2,6195-92-2
3-methyl-cyclopentanone

-
-
57-55-6,63625-56-9
propylene glycol

-
-
64-17-5
ethanol

-
-
623-37-0
n-hexan-3-ol

-
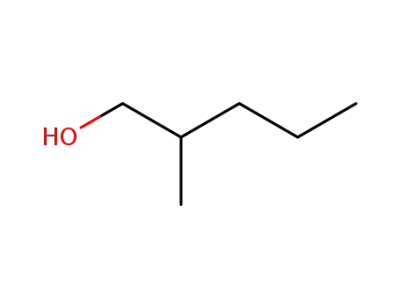
-
105-30-6
2-methylpentan-1-ol

-
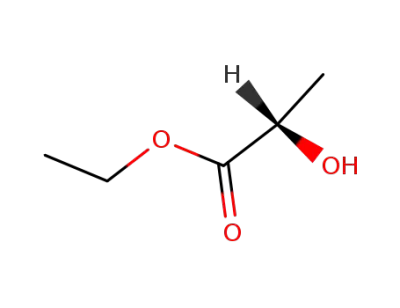
-
687-47-8
(S)-Ethyl lactate

-
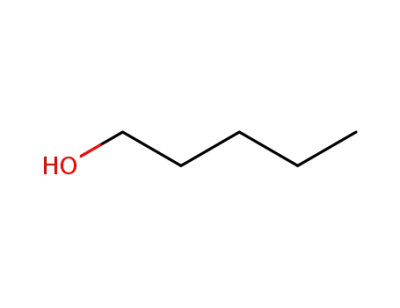
-
71-41-0
pentan-1-ol

-
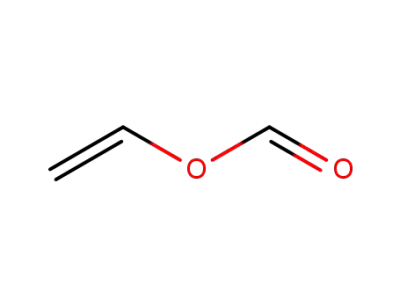
-
692-45-5
vinyl formate

-
-
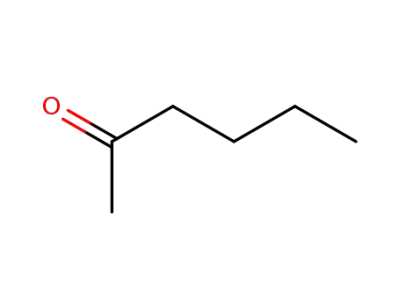
591-78-6
n-hexan-2-one

-
-
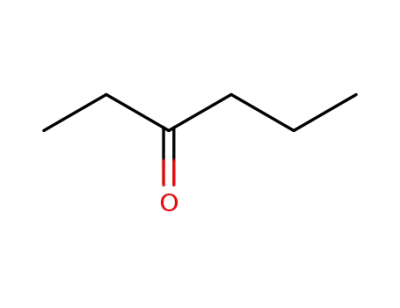
589-38-8
n-hexan-3-one

-
-
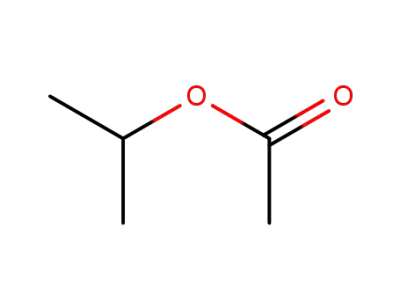
108-21-4
Isopropyl acetate

-
-
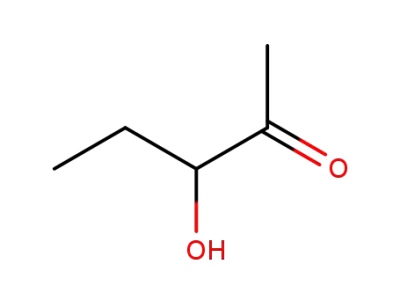
3142-66-3,113919-08-7
3-Hydroxy-2-pentanone

-
-
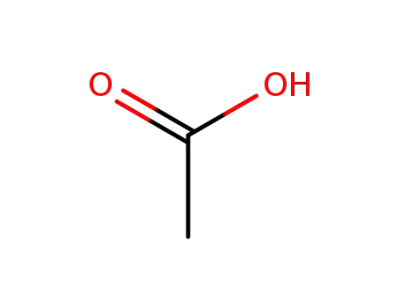
64-19-7,77671-22-8
acetic acid

-
-
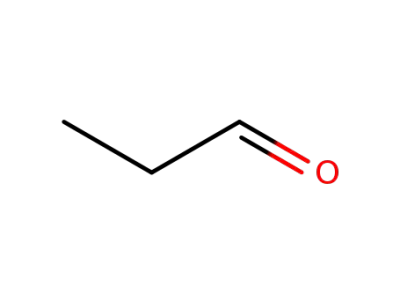
123-38-6
propionaldehyde

-
-
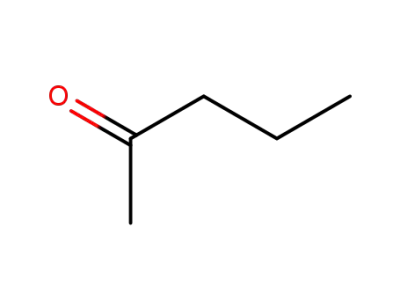
107-87-9
2-Pentanone

-
-
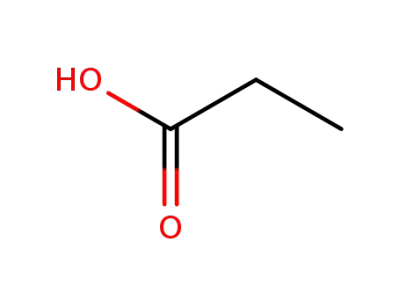
802294-64-0,79-09-4
propionic acid

-
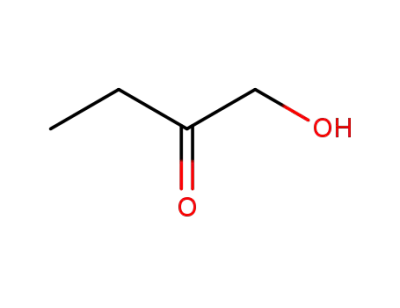
-
5077-67-8
1-Hydroxy-2-butanone

-

-
110-13-4
2,5-hexanedione

-

-
67-63-0,8013-70-5
isopropyl alcohol

-
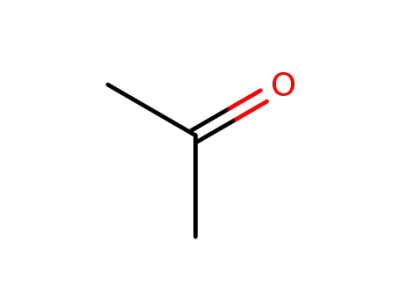
-
67-64-1
acetone

-
-
56-81-5,25618-55-7,64333-26-2,8013-25-0
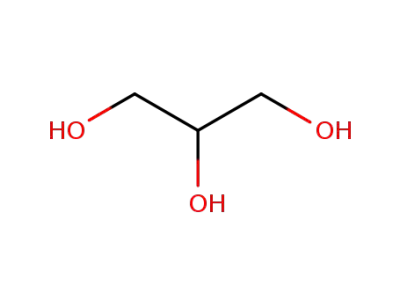
glycerol

-
-
96-22-0
pentan-3-one

-

-
79-31-2
isobutyric Acid

-
-
78-93-3
butanone

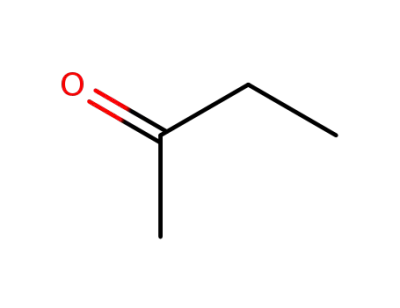
-
-
78-92-2,15892-23-6
iso-butanol

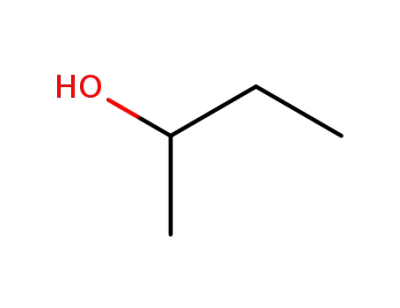
-
-
142-62-1
hexanoic acid

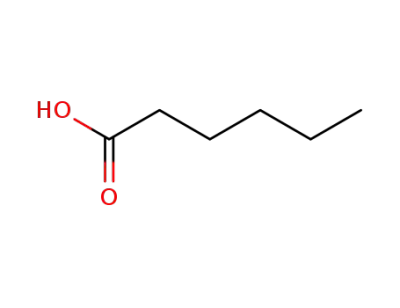
-
-
652-67-5
Isosorbide

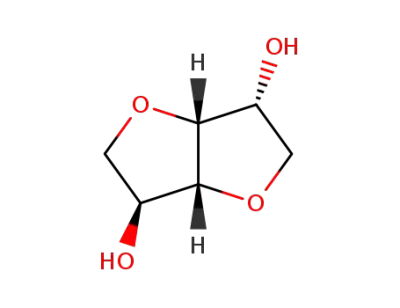
-
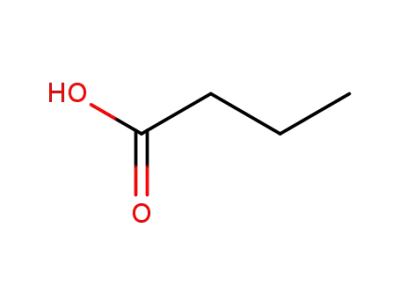
-
107-92-6
butyric acid

-
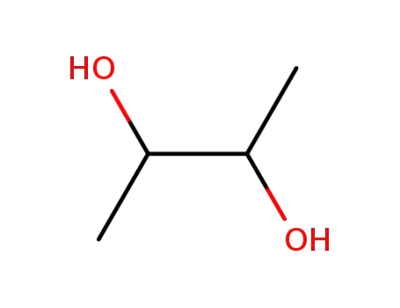
-
513-85-9
2.3-butanediol

-

-
111-27-3
hexan-1-ol

-
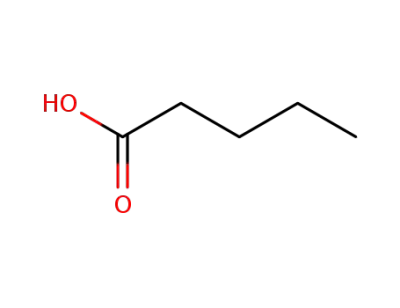
-
109-52-4
valeric acid
| Conditions | Yield |
|---|---|
|
platinum on carbon;
In
water;
for 3h;
Direct aqueous phase reforming;
|
79-31-2 Upstream products
-
71-23-8

propan-1-ol
-
64-18-6
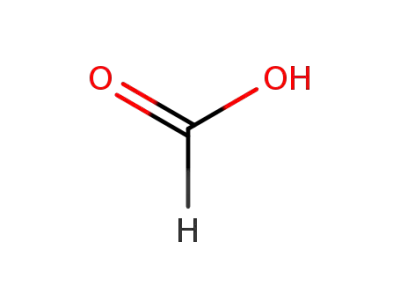
formic acid
-
56-23-5
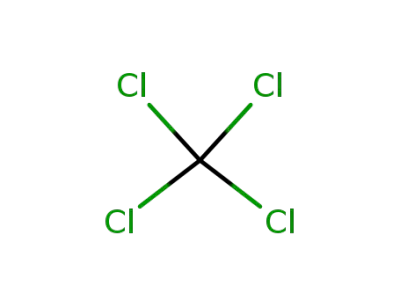
tetrachloromethane
-
117746-80-2
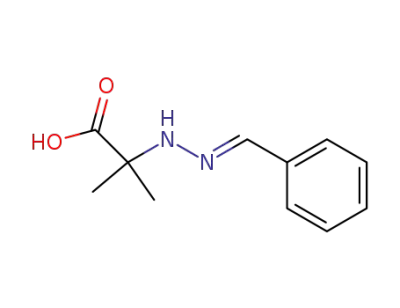
benzalhydrazone of hydrazinoisobutyric acid
79-31-2 Downstream products
-
2850-40-0
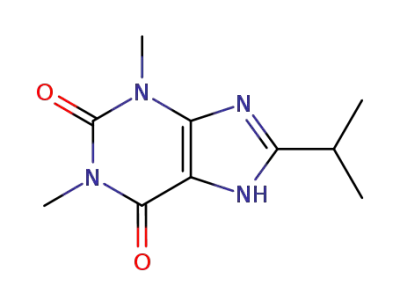
8-Isopropyl-theophyllin
-
7137-29-3

isobutyric acetic anhydride
-
6876-49-9
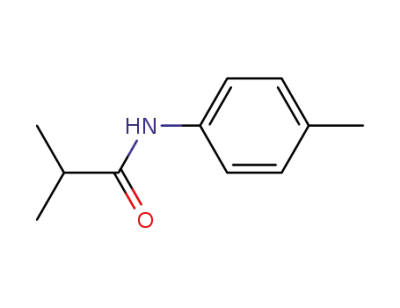
N-(4-methylphenyl)-2,2-dimethylacetamide
-
79-30-1

isobutyryl chloride
Relevant Products
-
Bismuth Octoate
CAS:67874-71-9
-
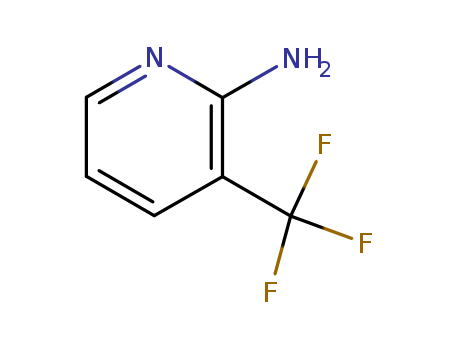
2-Amino-3-(trifluoromethyl)pyridine
CAS:183610-70-0
-
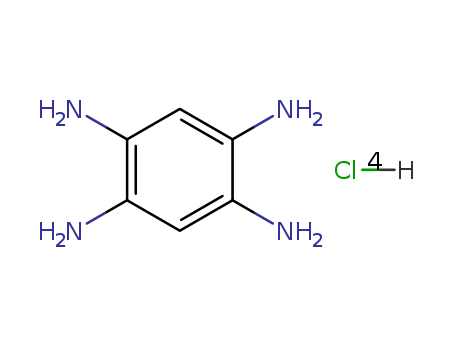
1,2,4,5-Benzenetetramine tetrahydrochloride
CAS:4506-66-5