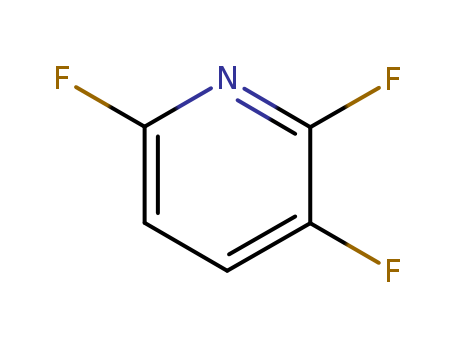
3512-18-3
- Product Name:2,3,6-Trifluoropyridine
- Molecular Formula:C5H2F3N
- Purity:99%
- Molecular Weight:133.073
Product Details
Good factory supply good 2,3,6-Trifluoropyridine 3512-18-3
- Molecular Formula:C5H2F3N
- Molecular Weight:133.073
- Appearance/Colour:colorless liquid
- Vapor Pressure:15.9mmHg at 25°C
- Melting Point:115-116 °C
- Refractive Index:1.42
- Boiling Point:123.7 °C at 760 mmHg
- PKA:-8.51±0.10(Predicted)
- Flash Point:28.6 °C
- PSA:12.89000
- Density:1.396 g/cm3
- LogP:1.49890
2,3,6-TRIFLUOROPYRIDINE(Cas 3512-18-3) Usage
InChI:InChI=1/C5H2F3N/c6-3-1-2-4(7)9-5(3)8/h1-2H
3512-18-3 Relevant articles
Rhodium catalyzed, carbon-hydrogen bond directed hydrodefluorination of fluoroarenes
Ekkert, Olga,Strudley, Sebastian D. A.,Rozenfeld, Alisa,White, Andrew J. P.,Crimmin, Mark R.
supporting information, p. 7027 - 7030 (2015/05/19)
[CpRhCl(μ-Cl)]2 is reported as a highly ...
Catalytic hydrodefluorination of fluoroaromatics with silanes as hydrogen source at a binuclear rhodium complex: Characterization of key intermediates
Zámostná, Lada,Ahrens, Mike,Braun, Thomas
, p. 132 - 142 (2013/10/01)
Stoichiometric and catalytic hydrodefluo...
Heterocyclic derivatives
-
, (2008/06/13)
The invention relates to heterocyclic de...
FLUORINATIONS WITH COMPLEX METAL FLUORIDES. PART 6. FLUORINATION OF PYRIDINE AND RELATED COMPOUNDS WITH CAESIUM TETRAFLUOROCOBALTATE(III)
Plevey, Raymond G.,Rendell, Richard W.,Tatlow, John Colin
, p. 159 - 170 (2007/10/02)
Pyridine has been fluorinated over caesi...
3512-18-3 Process route
-
-
2875-18-5
2,3,5,6-tetrafluoropyridine

-
-
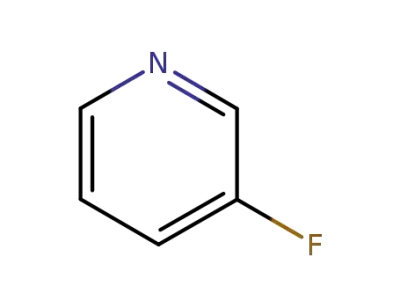
372-47-4
3-Fluoropyridine

-
-
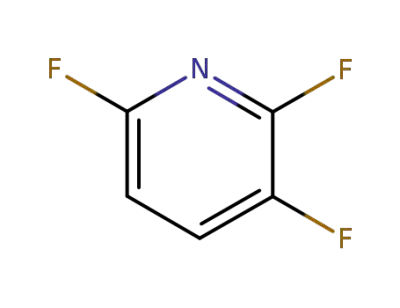
3512-18-3
2,3,6-trifluoropyridine

-
-
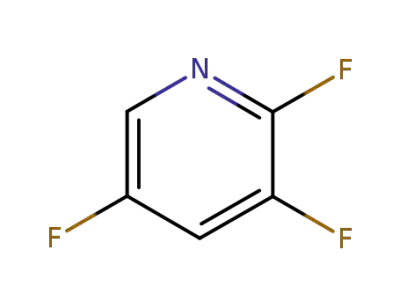
76469-41-5
2,3,5‐trifluoropyridine

-
-
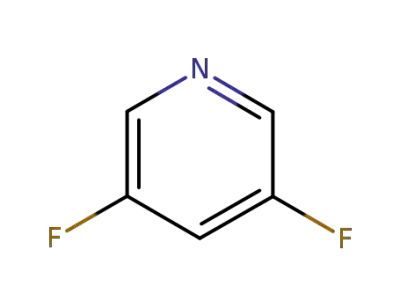
71902-33-5
3,5-difluoropyridine
| Conditions | Yield |
|---|---|
|
With
triethylsilane; [Rh(μ-H)(1,3-bis(diisopropylphosphanyl)propane)]2;
In
benzene-d6;
at 50 ℃;
for 48h;
regioselective reaction;
Inert atmosphere;
|
24 %Spectr. 7 %Spectr. 15 %Spectr. 8 %Spectr. |
-
-
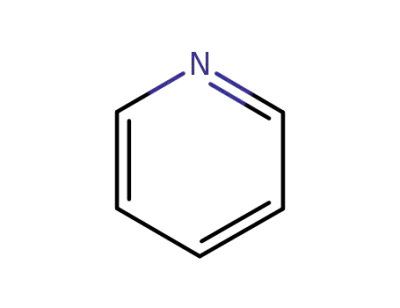
110-86-1
pyridine

-
-
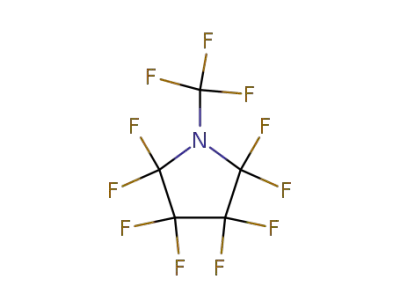
2344-10-7
perfluoro-N-ethylpyrrolidine

-

-
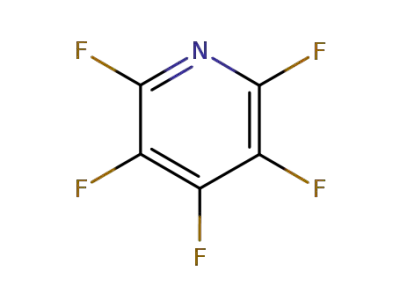
3512-18-3
2,3,6-trifluoropyridine

-
-
700-16-3
Pentafluoropyridine

-

-
371-77-7
N,N-Bis(trifluoromethyl)amine
| Conditions | Yield |
|---|---|
|
With
caesium tetrafluorocobaltate(III);
In
gaseous matrix;
at 310 ℃;
for 3.5h;
Further byproducts given;
|
35.3% 13.6% 8.2% 3.2% |
3512-18-3 Upstream products
-
110-86-1

pyridine
-
1333-74-0
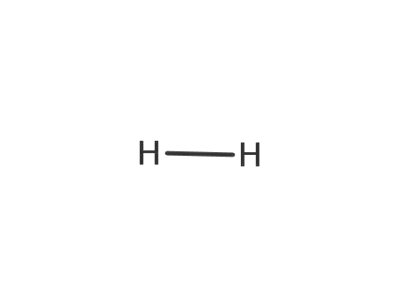
hydrogen
-
1735-84-8
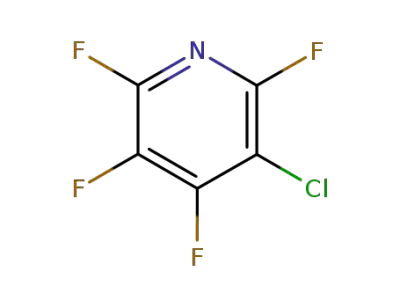
3-chloro-2,4,5,6-tetrafluoropyridine
-
2875-18-5

2,3,5,6-tetrafluoropyridine
3512-18-3 Downstream products
-
905587-08-8
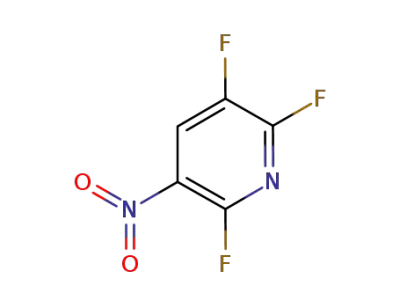
2,3,6-trifluoro-5-nitro-pyridine
-
944799-22-8
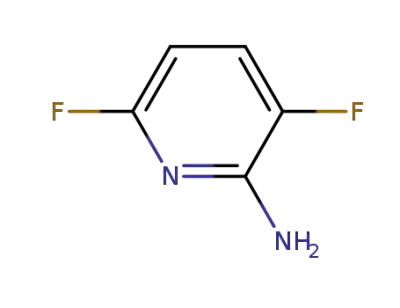
3,6-difluoropyridin-2-amine
-
1263374-84-0
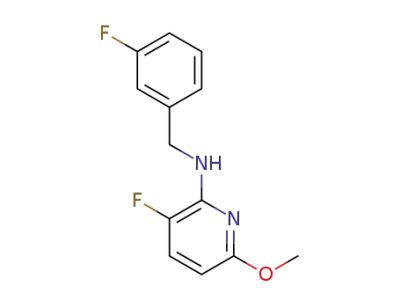
3-fluoro-N-(3-fluorobenzyl)-6-methoxypyridin-2-amine
-
1263374-85-1
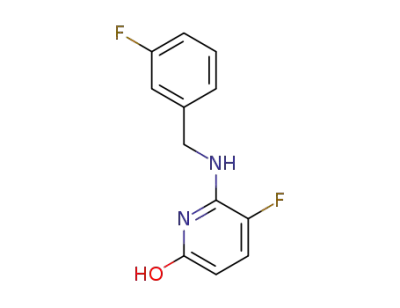
5-fluoro-6-(3-fluorobenzylamino)pyridine-2-ol
Relevant Products
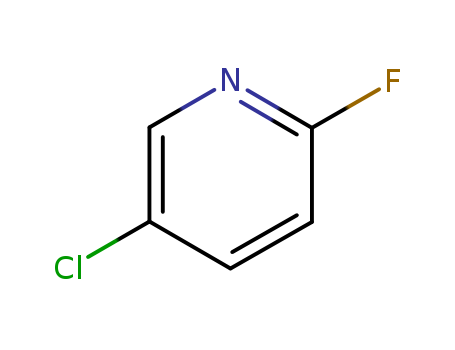
-
5-Chloro-2-fluoropyridine
CAS:1480-65-5
-
Dimethylamine hydrochloride
CAS:606-59-2
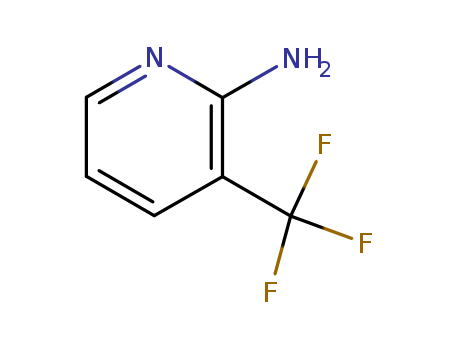
-
2-Amino-3-(trifluoromethyl)pyridine
CAS:183610-70-0