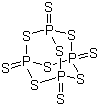
1314-80-3
- Product Name:Phosphorus pentasulfide
- Molecular Formula:P4S10
- Purity:99%
- Molecular Weight:444.54
Product Details
Bulk supply high purity Phosphorus pentasulfide 1314-80-3, Paid sample available
- Molecular Formula:P4S10
- Molecular Weight:444.54
- Appearance/Colour:yellow solid
- Melting Point:288 °C (561 K)
- Refractive Index:1.805
- Boiling Point:514 °C (787 K)
- PSA:221.94000
- Density:2.09 g/cm3
- LogP:4.96340
Phosphorus pentasulfide(Cas 1314-80-3) Usage
|
Air & Water Reactions |
Highly flammable. May heat and spontaneously ignite in presence of moisture [Haz. Chem. Data. 1969]. Reaction with water forms toxic hydrogen sulfide gas and phosphoric acid. Dangerous when wet. |
|
Reactivity Profile |
PHOSPHORUS PENTASULFIDE reacts vigorously with strong oxidants. Exothermically violent decomposition reaction with water, steam or acids to produce irritating fumes of phosphorus pentaoxide and highly toxic hydrogen sulfide gas. May ignite in contact with limited amounts of water [Haz. Chem. Data, 1975, p. 239]. Can be ignited by sparks or friction and its dust presents an explosion hazard in air at sufficient concentrations. Hydrogen sulfide gas evolved from reactions with water may also form explosive mixtures with air. |
|
Health Hazard |
Phosphorus pentasulfide is an irritant to the skin and eyes. Inhalation of its vapors can cause irritation of the respiratory passage. Oral toxicity of Phosphorus pentasulfide in rats was found to be moderate.LD50 value, oral (rats): 389 mg/kg (NIOSH 1986)Phosphorus pentasulfide can readily produce highly toxic hydrogen sulfide in the presence of moisture. Other toxic products from its combustion are sulfur dioxide and phosphorus pentoxide (corrosive).. |
|
Fire Hazard |
Special Hazards of Combustion Products: Products of combustion include sulfur dioxide and phosphorus pentoxide, which are irritating, toxic and corrosive. |
|
Flammability and Explosibility |
Highlyflammable |
|
Safety Profile |
A poison by ingestion. A severe eye and skin irritant. Readily liberates toxic hydrogen sulfide and phosphorus pentoxide and evolves heat on contact with moisture. Dangerous fire hazard in the form of dust when exposed to heat or flame. Spontaneous heating in the presence of moisture. Moderate explosion hazard in solid form by spontaneous chemical reaction. Reacts with water, steam, or acids to produce toxic and flammable vapors; can react vigorously with oxidizing materials. Incompatible with air, alcohols, water. To fight fire, use CO2snow, dry chemical, or sand. Used as an intermedate in manufacturing lubricant addltives, insecticides, and fertilizer agents. When heated to decomposition it emits highly toxic fumes of SO, and PO,. See also HYDROGEN SULFIDE. |
|
Potential Exposure |
Phosphorus pentasulfide is used as an intermediate in the manufacture of lubricant additives; insecticides, flotation agents; lubricating oil; ignition compounds; and matches. It is also used to introduce sulfur into rubber, and organic chemicals, such as pharmaceuticals. |
|
Shipping |
UN1340 Phosphorus pentasulfate, free from yellow or white phosphorus, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material, 4.1-flammable solid. |
|
Purification Methods |
Purify P2S5 by extraction and crystallisation with CS2, using a Soxhlet extractor, and is heated in a CO2 atmosphere at 150o to remove solvent. It liberates H2S in moist air. HARMFUL VAPOURS. [Klements in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 568 1963.] |
|
Incompatibilities |
Flammable solid; dust may form explosive mixture with air. Contact with water forms phosphorus pentoxide and an explosive mixture of hydrogen sulfide with air. Phosphorus pentasulfide is incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, ammonia, strong acids, strong bases, alcohols. Reaction with water or moisture in the air releases heat, hydrogen sulfide (H2S), sulfur dioxide, and phosphoric acid. Pyrophoric hazard, may self-ignite in moist air. |
|
Waste Disposal |
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Decompose with water, forming phosphoric acid, sulfuric acid and hydrogen sulfide. Provisions must be made for scrubbing hydrogen sulfide emissions. The acids may then be neutralized and diluted slowly to solution of soda ash and slaked lime with stirring, then flush to sewer with large volumes of water. |
|
General Description |
A greenish yellow solid with an odor of rotten eggs that may paralyze the sense of smell at hazardous concentrations in air. Density 2.04 g / cm3. Phosphorus pentasulfide is used for making lube oil additives, insecticides, flotation agents, safety matches, blown asphalt, and other products and chemicals. |
InChI:InChI=1/H4O5P2S2/c1-6(2,8)5-7(3,4)9/h(H2,1,2,8)(H2,3,4,9)
Relevant Products
-
Pinacolone
CAS:75-97-8
-
2,3,5,6-Tetrafluoropyridine
CAS:2875-18-5