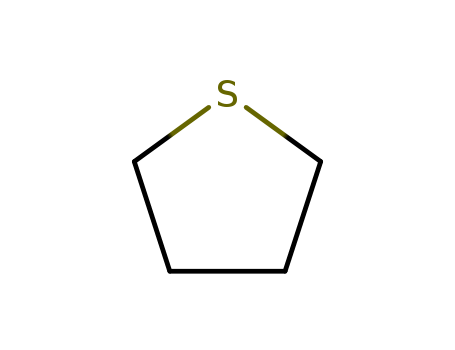
110-01-0
- Product Name:Tetrahydrothiophene
- Molecular Formula:C4H8S
- Purity:99%
- Molecular Weight:88.1735
Product Details
Cost-effective and customizable Tetrahydrothiophene 110-01-0 factory
- Molecular Formula:C4H8S
- Molecular Weight:88.1735
- Appearance/Colour:volatile, clear, colorless liquid with a strong unpleasant odor
- Vapor Pressure:18 mm Hg ( 25 °C)
- Melting Point:-96 °C
- Refractive Index:n20/D 1.504(lit.)
- Boiling Point:121.5 °C at 760 mmHg
- Flash Point:12.8 °C
- PSA:25.30000
- Density:0.999 g/cm3
- LogP:1.51340
Tetrahydrothiophene(Cas 110-01-0) Usage
|
Preparation |
Tetrahydrothiophene is prepared by the reaction of hydrogen sulfide and furan in the vapor phase at 400°C. This vapor-phase reaction is catalyzed by alumina(Al2O3) and other heterogenous acid catalysts. |
|
Air & Water Reactions |
Highly flammable. Insoluble in water. |
|
Reactivity Profile |
Organosulfides, such as Tetrahydrothiophene, are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Slow addition of hydrogen peroxide to the thiophene resulted in explosions on three occasions, Chem. Eng. News, 1974, 52(39), 3. |
|
Health Hazard |
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |
|
Fire Hazard |
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
|
Safety Profile |
Mildly toxic by inhalation. Flammable liquid. Potentially explosive reaction with hydrogen peroxide. When heated to decomposition it emits toxic fumes of SOx. |
|
Purification Methods |
The crude material is purified by crystallisation of the mercuric chloride complex to a constant melting point. It is then regenerated, washed, dried, and fractionally distilled. [Whitehead et al. J Am Chem Soc 73 3632 1951.] It has been dried over Na2SO4 and distilled in a vacuum [Roberts & Friend J Am Chem Soc 108 7204 1986]. [Beilstein 17 I 5, 17 II 15, 17 III/IV 34, 17/1 V 36.] |
|
General Description |
A water-white liquid. About the same density as water and insoluble in water. Flash point near 20°F. Vapors heavier than air. Used as a solvent and to make other chemicals. |
InChI:InChI=1/C4H8S/c1-2-4-5-3-1/h1-4H2
110-01-0 Relevant articles
Visible-Light-Promoted Oxo-difluoroalkylation of Alkenes with DMSO as the Oxidant
Xia, Zi-Hao,Gao, Zhong-Hua,Dai, Lei,Ye, Song
, p. 7388 - 7394 (2019)
Visible-light-promoted oxo-difluoroalkyl...
Six-coordinate nitrosyl and nitro complexes of meso - Tetratolylporphyrinatocobalt with trans sulfur-donor ligands
Kurtikyan, Tigran S.,Gulyan, Gurgen M.,Dalaloyan, Arina M.,Kidd, Bryce E.,Goodwin, John A.
, p. 7793 - 7798 (2010)
By Fourier transform infrared and optica...
The reactivity of (Me3Si)3SiH with sulfoxides under free radical conditions
Chatgilialoglu, Chryssostomos,Ferreri, Carla
, p. 7764 - 7769 (2016)
The radical-initiated reaction of (Me3Si...
ELECTROREDUCTION OF THIOPHENE BY ORGANIC ELECTRON CARRIERS AND EFFECT OF PROTON DONORS
Mairanovskii, S. G.,Kosychenko, L. I.,Taits, S. Z.
, p. 995 - 997 (1980)
-
-
Olah et al.
, p. 4503 (1978)
-
Characterization and catalytic performance of {Mo2O2S2}-based oxothiomolybdenum cyclic clusters supported on mesoporous SBA-15
Xin, Zhifeng,Wei, Wei,Chen, Min,Jia, Ai-Quan,Zhang, Qian-Feng
, p. 86 - 90 (2015)
Cyclic polyoxothiomolybdate clusters con...
-
Alper,Keung
, p. 53 (1970)
-
-
Gardner et al.
, p. 1419,1420 (1973)
-
-
Nojima et al.
, p. 1343 (1975)
-
-
Greenfield et al.
, p. 1054 (1958)
-
Synthesis, structure, and reactions of a copper-sulfido cluster comprised of the parent Cu2S unit: {(NHC)Cu}2(μ-S)
Zhai, Junjie,Filatov, Alexander S.,Hillhouse, Gregory L.,Hopkins, Michael D.
, p. 589 - 595 (2016)
The synthesis of the first CuI2(μ-S) com...
-
Durst et al.
, p. 1777 (1974)
-
-
Johnson et al.
, p. 919 (1972)
-
The synthesis of polymeric sulfides by reaction of dihaloalkanes with sodium sulfide
Smith, Keith,El-Hiti, Gamal A.,Al-Zuhairi, Ali J.
, p. 521 - 531 (2011)
Several poly(alkylene sulfide)s have bee...
Coenzyme F430 from Methanogenic Bacteria: Methane Formation by Reductive Carbon-Sulphur Bond Cleavage of Methyl Sulphonium Ions Catalysed by F430 Pentamethyl Ester
Jaun, Bernhard,Pfaltz, Andreas
, p. 293 - 294 (1988)
The nickel(I)-form of coenzyme F430 pent...
Neutral and Anionic Binuclear Perhalogenophenyl Platinum-Silver Complexes with Pt->Ag Bonds Unsupported by Covalent Bridges. Molecular Structures of , and (tht = tetrahydrothiophene)
Uson, Rafael,Fornies, Juan,Tomas, Milagros,Ara, Irene,Casas, Jose M.,Martin, Antonio
, p. 2253 - 2264 (1991)
Heterobinulear complexes of general form...
Efficient reduction of sulfoxides with 2,6-dihydroxypyridine
Miller, Samantha J.,Collier, Talia R.,Wu, Weiming
, p. 3781 - 3783 (2000)
2,6-Dihydroxypyridine was found to be an...
Formation of active sites and hydrodesulfurization activity of rhodium phosphide catalyst: Effect of reduction temperature and phosphorus loading
Kanda, Yasuharu,Temma, Chisato,Sawada, Ayaka,Sugioka, Masatoshi,Uemichi, Yoshio
, p. 410 - 419 (2014)
Effects of reduction temperature and pho...
Catalytic dehydrogenation of amines to imines and the in-situ reduction of sulfoxides into sulfides
Li, Bo,Liu, Bing,Liu, Xixi,Wang, Wei,Wang, Yanxin,Xiang, Nian,Zhang, Zehui
, p. 81 - 88 (2021/07/30)
The catalytic acceptorless dehydrogenati...
Preparation method of N-(4-methylpyridine-3-yl) methyl carbamate
-
Paragraph 0020; 0022; 0025-0026; 0030; 0032; 0035-0036, (2021/06/02)
The invention relates to the technical f...
Lewis Acidic Boranes, Lewis Bases, and Equilibrium Constants: A Reliable Scaffold for a Quantitative Lewis Acidity/Basicity Scale
Mayer, Robert J.,Hampel, Nathalie,Ofial, Armin R.
supporting information, p. 4070 - 4080 (2021/01/29)
A quantitative Lewis acidity/basicity sc...
Photocatalytic Deoxygenation of Sulfoxides Using Visible Light: Mechanistic Investigations and Synthetic Applications
Clarke, Aimee K.,Parkin, Alison,Rossi-Ashton, James A.,Taylor, Richard J. K.,Unsworth, William P.
, p. 5814 - 5820 (2020/07/21)
The photocatalytic deoxygenation of sulf...
110-01-0 Process route
-
-
188290-36-0,8014-23-1,25233-34-5
thiophene

-
-
110-01-0
thiophene

-
-
1708-32-3
2,5-dihydro-thiophene
| Conditions | Yield |
|---|---|
|
With
biphenyl; tetraethylammonium perchlorate;
In
water; N,N-dimethyl-formamide;
electrolysis in a separated cell, the operating electrode was mercury, the reference electrode was the saturated calomel electrode, initial current was 150 mA;
|
24% 19.7% |
|
With
biphenyl; tetraethylammonium perchlorate;
In
water; N,N-dimethyl-formamide;
electrolysis in a separated cell, the operating electrode was mercury, the reference electrode was the saturated calomel electrode, initial current was 150 mA;
|
19.7% 24% |
|
With
biphenyl; tetraethylammonium perchlorate;
In
water; N,N-dimethyl-formamide;
Product distribution;
electrolysis in a separated cell, the operating electrode was mercury, the reference electrode was the saturated calomel electrode, initial current 150 mA; other solvent;
|
24% 19.7% |
-

-
159551-96-9
1-(tert-butyldithiobutylcarbonyloxy)pyridine-2(1H)-thione

-

-
110-01-0
thiophene

-
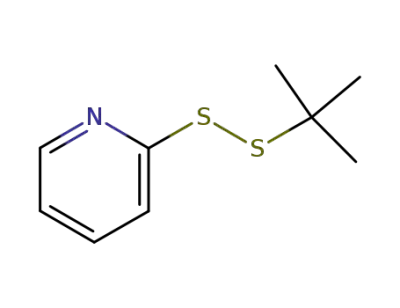
-
24367-44-0
2-(2-tert-butyldisulfanyl)pyridine
| Conditions | Yield |
|---|---|
|
In
benzene;
at 80 ℃;
for 2h;
Irradiation;
|
110-01-0 Upstream products
-
109-99-9
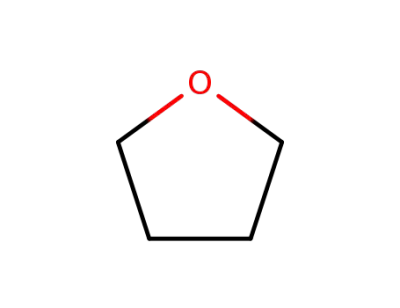
tetrahydrofuran
-
1708-32-3

2,5-dihydro-thiophene
-
188290-36-0

thiophene
-
110-52-1

1,4-dibromo-butane
110-01-0 Downstream products
-
1600-44-8
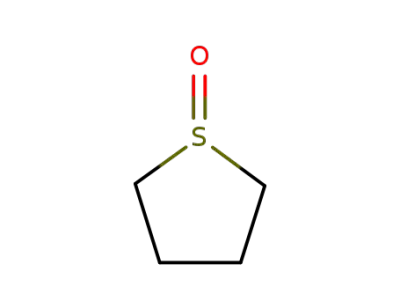
1-oxothiolane
-
1608-66-8
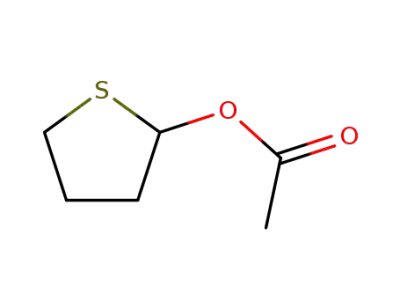
2-(acetoxy)tetrahydrothiophene
-
108-24-7
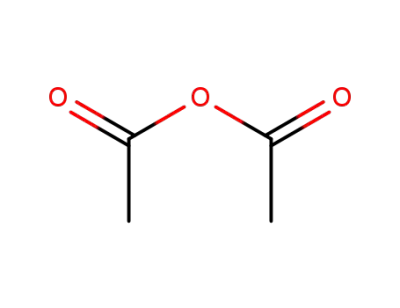
acetic anhydride
-
64-19-7

acetic acid
Relevant Products
-
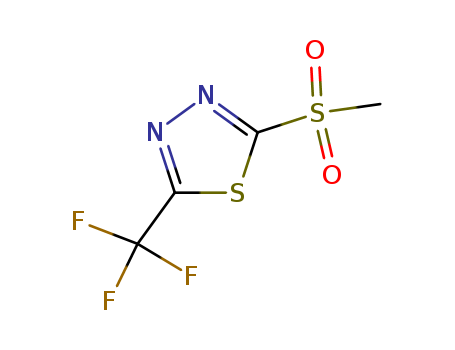
2-(Methylsulfonyl)-5-(Trifluoromethyl)-1,3,4-Thiadiazol
CAS:27603-25-4
-
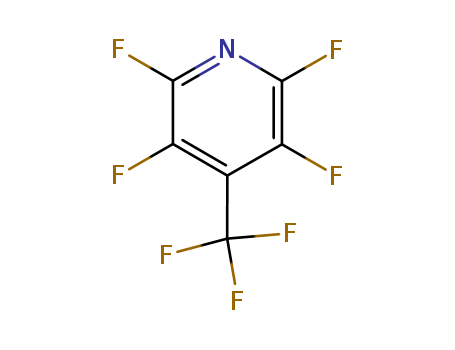
2,3,5,6-tetrafluoro-4-(trifluoromethyl)pyridine
CAS:3244-44-8
-
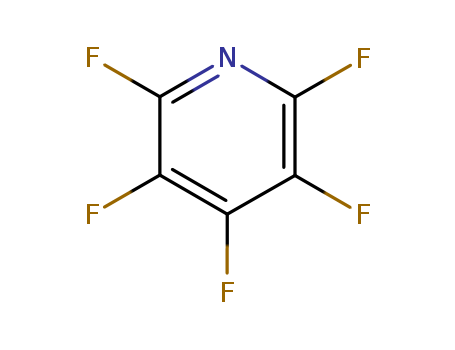
Pentafluoropyridine
CAS:700-16-3